Probing the dynamics of doxorubicin-DNA intercalation during the initial activation of apoptosis by fluorescence lifetime imaging microscopy (FLIM)
- PMID: 23028696
- PMCID: PMC3445590
- DOI: 10.1371/journal.pone.0044947
Probing the dynamics of doxorubicin-DNA intercalation during the initial activation of apoptosis by fluorescence lifetime imaging microscopy (FLIM)
Erratum in
- PLoS One. 2012;7(11). doi:10.1371/annotation/4c43c8c8-0a4e-425b-a72f-74e84f6f3c28
Abstract
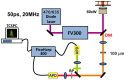
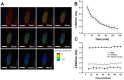
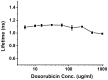
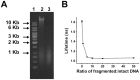
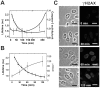
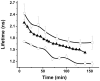
Doxorubicin is a potent anthracycline antibiotic, commonly used to treat a wide range of cancers. Although postulated to intercalate between DNA bases, many of the details of doxorubicin's mechanism of action remain unclear. In this work, we demonstrate the ability of fluorescence lifetime imaging microscopy (FLIM) to dynamically monitor doxorubicin-DNA intercalation during the earliest stages of apoptosis. The fluorescence lifetime of doxorubicin in nuclei is found to decrease rapidly during the first 2 hours following drug administration, suggesting significant changes in the doxorubicin-DNA binding site's microenvironment upon apoptosis initiation. Decreases in doxorubicin fluorescence lifetimes were found to be concurrent with increases in phosphorylation of H2AX (an immediate signal of DNA double-strand breakage), but preceded activation of caspase-3 (a late signature of apoptosis) by more than 150 minutes. Time-dependent doxorubicin FLIM analyses of the effects of pretreating cells with either Cyclopentylidene-[4-(4-chlorophenyl)thiazol-2-yl)-hydrazine (a histone acetyltransferase inhibitor) or Trichostatin A (a histone deacetylase inhibitor) revealed significant correlation of fluorescence lifetime with the stage of chromatin decondensation. Taken together, our findings suggest that monitoring the dynamics of doxorubicin fluorescence lifetimes can provide valuable information during the earliest phases of doxorubicin-induced apoptosis; and implicate that FLIM can serve as a sensitive, high-resolution tool for the elucidation of intercellular mechanisms and kinetics of anti-cancer drugs that bear fluorescent moieties.
Conflict of interest statement
Figures

References
-
- Blum RH, Carter SK (1974) Adriamycin, A new anticancer drug with significant clinical activity. Ann. Intern. Med. 80(1): 249–259. - PubMed
-
- Carter SK (1975) Adriamycin – thoughts for the future. Cancer Chemother. Rep. 63: 1877–1883.
-
- Hortobagyi GN (1997) Anthracyclines in the treatment of cancer an overview. Drug 54: 1–7. - PubMed
-
- Li ZX, Wang TT, Wu YT, Xu CM, Dong MY, et al. (2008) Adriamycin induces H2AX phosphorylation in human spermatozoa. Asian J Androl 10(5): 749–757. - PubMed
-
- Hurley LH (2002) DNA and its associated processes as targets for cancer therapy. Nature Reviews Cancer 2: 188–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

