NVX-412, a new oncology drug candidate, induces S-phase arrest and DNA damage in cancer cells in a p53-independent manner
- PMID: 23028738
- PMCID: PMC3441706
- DOI: 10.1371/journal.pone.0045015
NVX-412, a new oncology drug candidate, induces S-phase arrest and DNA damage in cancer cells in a p53-independent manner
Abstract
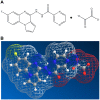
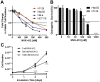
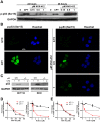
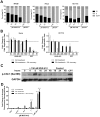
The new molecular entity quinoxalinhydrazide derivative NVX-412 was identified as a promising drug candidate for the treatment of various cancer types due to its strong cytotoxic activity and relative specificity. Here, we provide first data about the mechanisms of action of NVX-412. We show that NVX-412 exerts its anti-neoplastic activity in a p53-independent manner and induces S-phase arrest and DNA damage as assessed by γH2AX staining. We suggest a bi-modal (dose-dependent) mode of action of NVX-412, being primarily cytostatic at lower and predominantly cytotoxic at higher concentrations. Based on the broad and consistent anti-neoplastic activity observed, NVX-412 holds promise as an effective drug candidate for the treatment of various cancer types, especially for hematological malignancies with highly unmet medical need.
Conflict of interest statement
Figures

References
-
- WHO (2011) Causes of Death 2008, Summary Tables.
-
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3–26. - PubMed
-
- Grande F, Aiello F, Grazia OD, Brizzi A, Garofalo A, et al. (2007) Synthesis and antitumor activities of a series of novel quinoxalinhydrazides. Bioorg Med Chem 15: 288–294. - PubMed
-
- Aakeroy CB, Fasulo ME, Desper J (2007) Cocrystal or salt: does it really matter? Mol Pharm 4: 317–322. - PubMed
-
- Shoemaker RH (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6: 813–823. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

