Sirt5 deacylation activities show differential sensitivities to nicotinamide inhibition
- PMID: 23028781
- PMCID: PMC3446968
- DOI: 10.1371/journal.pone.0045098
Sirt5 deacylation activities show differential sensitivities to nicotinamide inhibition
Abstract
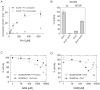
Sirtuins are protein deacylases regulating metabolism and aging processes, and the seven human isoforms are considered attractive therapeutic targets. Sirtuins transfer acyl groups from lysine sidechains to ADP-ribose, formed from the cosubstrate NAD(+) by release of nicotinamide, which in turn is assumed to be a general Sirtuin inhibitor. Studies on Sirtuin regulation have been hampered, however, by shortcomings of available assays. Here, we describe a mass spectrometry-based, quantitative deacylation assay not requiring any substrate labeling. Using this assay, we show that the deacetylation activity of human Sirt5 features an unusual insensitivity to nicotinamide inhibition. In contrast, we find similar values for Sirt5 and Sirt3 for the intrinsic NAD(+) affinity as well as the apparent NAD(+) affinity in presence of peptide. Structure comparison and mutagenesis identify an Arg neighboring to the Sirt5 nicotinamide binding pocket as a mediator of nicotinamide resistance, and statistical sequence analyses along with testing further Sirtuins reveal a network of coevolved residues likely defining a nicotinamide-insensitive Sirtuin deacetylase family. The same Arg was recently reported to render Sirt5 a preferential desuccinylase, and we find that this Sirt5 activity is highly sensitive to nicotinamide inhibition. Analysis of Sirt5 structures and activity data suggest that an Arg/succinate interaction is the molecular basis of the differential nicotinamide sensitivities of the two Sirt5 activities. Our results thus indicate a Sirtuin subfamily with nicotinamide-insensitive deacetylase activity and suggest that the molecular features determining nicotinamide sensitivity overlap with those dominating deacylation specificity, possibly suggesting that other subfamily members might also prefer other acylations than acetylations.
Conflict of interest statement
Figures




References
-
- Guarente L, Picard F (2005) Calorie restriction - the SIR2 connection. Cell 120: 473–482. - PubMed
-
- Sauve AA, Wolberger C, Schramm VL, Boeke JD (2006) The biochemistry of sirtuins. Annu Rev Biochem 75: 435–465. - PubMed
-
- Milne JC, Denu JM (2008) The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol 12: 11–17. - PubMed
-
- Lakshminarasimhan M, Steegborn C (2011) Emerging mitochondrial signaling mechanisms in physiology, aging processes, and as drug targets. Exp Gerontol 46: 174–177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

