Opc expression, LPS immunotype switch and pilin conversion contribute to serum resistance of unencapsulated meningococci
- PMID: 23028802
- PMCID: PMC3447861
- DOI: 10.1371/journal.pone.0045132
Opc expression, LPS immunotype switch and pilin conversion contribute to serum resistance of unencapsulated meningococci
Abstract
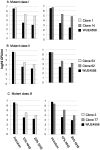
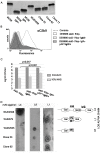
Neisseria meningitidis employs polysaccharides and outer membrane proteins to cope with human serum complement attack. To screen for factors influencing serum resistance, an assay was developed based on a colorimetric serum bactericidal assay. The screening used a genetically modified sequence type (ST)-41/44 clonal complex (cc) strain lacking LPS sialylation, polysaccharide capsule, the factor H binding protein (fHbp) and MutS, a protein of the DNA repair mechanism. After killing of >99.9% of the bacterial cells by serum treatment, the colorimetric assay was used to screen 1000 colonies, of which 35 showed enhanced serum resistance. Three mutant classes were identified. In the first class of mutants, enhanced expression of Opc was identified. Opc expression was associated with vitronectin binding and reduced membrane attack complex deposition confirming recent observations. Lipopolysaccharide (LPS) immunotype switch from immunotype L3 to L8/L1 by lgtA and lgtC phase variation represented the second class. Isogenic mutant analysis demonstrated that in ST-41/44 cc strains the L8/L1 immunotype was more serum resistant than the L3 immunotype. Consecutive analysis revealed that the immunotypes L8 and L1 were frequently observed in ST-41/44 cc isolates from both carriage and disease. Immunotype switch to L8/L1 is therefore suggested to contribute to the adaptive capacity of this meningococcal lineage. The third mutant class displayed a pilE allelic exchange associated with enhanced autoaggregation. The mutation of the C terminal hypervariable region D of PilE included a residue previously associated with increased pilus bundle formation. We suggest that autoaggregation reduced the surface area accessible to serum complement and protected from killing. The study highlights the ability of meningococci to adapt to environmental stress by phase variation and intrachromosomal recombination affecting subcapsular antigens.
Conflict of interest statement
Figures






Similar articles
-
Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides.Mol Microbiol. 1995 Nov;18(4):741-54. doi: 10.1111/j.1365-2958.1995.mmi_18040741.x. Mol Microbiol. 1995. PMID: 8817495
-
The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis.Microb Pathog. 1992 Sep;13(3):219-24. doi: 10.1016/0882-4010(92)90022-g. Microb Pathog. 1992. PMID: 1283998
-
Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity.Infect Immun. 1994 Dec;62(12):5290-5. doi: 10.1128/iai.62.12.5290-5295.1994. Infect Immun. 1994. PMID: 7960107 Free PMC article.
-
Short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis of potential value for production of outer membrane vesicle vaccines.Microb Pathog. 1995 Sep;19(3):159-68. doi: 10.1006/mpat.1995.0054. Microb Pathog. 1995. PMID: 8559044
-
The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis.Mol Immunol. 1999 Sep-Oct;36(13-14):915-28. doi: 10.1016/s0161-5890(99)00114-5. Mol Immunol. 1999. PMID: 10698346 Review.
Cited by
-
Hijacking Complement Regulatory Proteins for Bacterial Immune Evasion.Front Microbiol. 2016 Dec 20;7:2004. doi: 10.3389/fmicb.2016.02004. eCollection 2016. Front Microbiol. 2016. PMID: 28066340 Free PMC article. Review.
-
Conserved Patterns of Microbial Immune Escape: Pathogenic Microbes of Diverse Origin Target the Human Terminal Complement Inhibitor Vitronectin via a Single Common Motif.PLoS One. 2016 Jan 25;11(1):e0147709. doi: 10.1371/journal.pone.0147709. eCollection 2016. PLoS One. 2016. PMID: 26808444 Free PMC article.
-
Phase variation mediates reductions in expression of surface proteins during persistent meningococcal carriage.Infect Immun. 2014 Jun;82(6):2472-84. doi: 10.1128/IAI.01521-14. Epub 2014 Mar 31. Infect Immun. 2014. PMID: 24686058 Free PMC article.
-
How the Knowledge of Interactions between Meningococcus and the Human Immune System Has Been Used to Prepare Effective Neisseria meningitidis Vaccines.J Immunol Res. 2015;2015:189153. doi: 10.1155/2015/189153. Epub 2015 Aug 17. J Immunol Res. 2015. PMID: 26351643 Free PMC article. Review.
-
Meningococcal disease and the complement system.Virulence. 2014 Jan 1;5(1):98-126. doi: 10.4161/viru.26515. Epub 2013 Oct 8. Virulence. 2014. PMID: 24104403 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

