Deletion of IL-33R (ST2) abrogates resistance to EAE in BALB/C mice by enhancing polarization of APC to inflammatory phenotype
- PMID: 23028861
- PMCID: PMC3445483
- DOI: 10.1371/journal.pone.0045225
Deletion of IL-33R (ST2) abrogates resistance to EAE in BALB/C mice by enhancing polarization of APC to inflammatory phenotype
Abstract
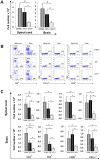
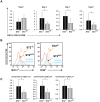
The administration of interleukin 33 and deletion of IL-33 receptor, ST2 molecule, affects the induction of autoimmunity in different experimental models of human autoimmune diseases. The aim of this study was to analyze the effect of ST2 deletion on the induction of experimental autoimmune encephalomyelitis (EAE) in resistant BALB/c mice. Mice were immunized with MOG(35-55) peptide or disease was induced by passive transfer of encephalitogenic singenic cells and EAE was clinically and histologically evaluated. Expression of intracellular inflammatory cytokines, markers of activation and chemokine receptors on lymphoid tissue and CNS infiltrating mononuclear cells was analyzed by flow cytometry. We report here that deletion of ST2(-/-) molecule abrogates resistance of BALB/c mice to EAE induction based on clinical and histopathological findings. Brain and spinal cord infiltrates of ST2(-/-) mice had significantly higher number of CD4(+) T lymphocytes containing inflammatory cytokines compared to BALB/c WT mice. Adoptive transfer of ST2(-/-) primed lymphocytes induced clinical signs of the disease in ST2(-/-) as well as in WT mice. MOG(35-55) restimulated ST2(-/-) CD4(+) cells as well as ex vivo analyzed lymph node cells had higher expression of T-bet and IL-17, IFN-γ, TNF-α and GM-CSF in comparison with WT CD4(+) cells. ST2(-/-) mice had higher percentages of CD4(+) cells expressing chemokine receptors important for migration to CNS in comparison with WT CD4(+) cells. Draining lymph nodes of ST2(-/-) mice contained higher percentage of CD11c(+)CD11b(+)CD8(-) cells containing inflammatory cytokines IL-6 and IL-12 with higher expression of activation markers. Transfer of ST2(-/-) but not WT dendritic cells induced EAE in MOG(35-55) immunized WT mice. Our results indicate that ST2 deficiency attenuates inherent resistance of BALB/c mice to EAE induction by enhancing differentiation of proinflammatory antigen presenting cells and consecutive differentiation of encephalitogenic T cells in the draining lymph node rather than affecting their action in the target tissue.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

