Distinct motifs in the intracellular domain of human CD30 differentially activate canonical and alternative transcription factor NF-κB signaling
- PMID: 23028875
- PMCID: PMC3445475
- DOI: 10.1371/journal.pone.0045244
Distinct motifs in the intracellular domain of human CD30 differentially activate canonical and alternative transcription factor NF-κB signaling
Abstract
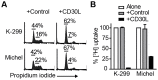
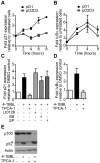
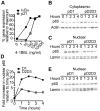
The TNF-receptor superfamily member CD30 is expressed on normal and malignant lymphocytes, including anaplastic large cell lymphoma (ALCL) cells. CD30 transmits multiple effects, including activation of NF-κB signaling, cell proliferation, growth arrest and apoptosis. How CD30 generates these pleiotropic effects is currently unknown. Herein we describe ALCL cells expressing truncated forms of the CD30 intracellular domain that allowed us to identify the key regions responsible for transmitting its biological effects in lymphocytes. The first region (CD30(519-537)) activated both the alternative and canonical NF-κB pathways as detected by p100 and IκBα degradation, IKKβ-dependent transcription of both IκBα and the cyclin-dependent kinase inhibitor p21(WAF1/CIP1) and induction of cell cycle arrest. In contrast, the second region of CD30 (CD30(538-595)) induced some aspects of canonical NF-κB activation, including transcription of IκBα, but failed to activate the alternative NF-κB pathway or drive p21(WAF1/CIP1)-mediated cell-cycle arrest. Direct comparison of canonical NF-κB activation by the two motifs revealed 4-fold greater p65 nuclear translocation following CD30(519-537) engagement. These data reveal that independent regions of the CD30 cytoplasmic tail regulate the magnitude and type of NF-κB activation and additionally identify a short motif necessary for CD30-driven growth arrest signals in ALCL cells.
Conflict of interest statement
Figures


References
-
- Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, et al. (1995) CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 85: 1–14. - PubMed
-
- Al-Shamkhani A (2004) The role of CD30 in the pathogenesis of haematopoietic malignancies. Curr Opin Pharmacol 4: 355–359. - PubMed
-
- Telford WG, Nam SY, Podack ER, Miller RA (1997) CD30-regulated apoptosis in murine CD8 T cells after cessation of TCR signals. Cell Immunol 182: 125–136. - PubMed
-
- Dai Z, Nasr IW, Reel M, Deng S, Diggs L, et al. (2005) Impaired recall of CD8 memory T cells in immunologically privileged tissue. J Immunol 174: 1165–1170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

