The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus
- PMID: 23028880
- PMCID: PMC3445467
- DOI: 10.1371/journal.pone.0045250
The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus
Abstract
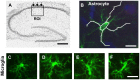
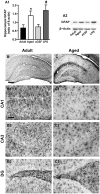
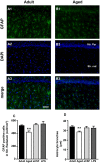
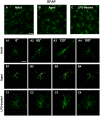
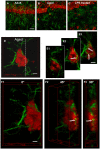
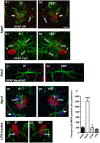
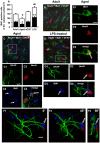
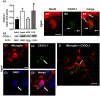
Ageing is accompanied by a decline in cognitive functions; along with a variety of neurobiological changes. The association between inflammation and ageing is based on complex molecular and cellular changes that we are only just beginning to understand. The hippocampus is one of the structures more closely related to electrophysiological, structural and morphological changes during ageing. In the present study we examined the effect of normal ageing and LPS-induced inflammation on astroglia-neuron interaction in the rat hippocampus of adult, normal aged and LPS-treated adult rats. Astrocytes were smaller, with thicker and shorter branches and less numerous in CA1 Str. radiatum of aged rats in comparison to adult and LPS-treated rats. Astrocyte branches infiltrated apoptotic neurons of aged and LPS-treated rats. Cellular debris, which were more numerous in CA1 of aged and LPS-treated rats, could be found apposed to astrocytes processes and were phagocytated by reactive microglia. Reactive microglia were present in the CA1 Str. Radiatum, often in association with apoptotic cells. Significant differences were found in the fraction of reactive microglia which was 40% of total in adult, 33% in aged and 50% in LPS-treated rats. Fractalkine (CX3CL1) increased significantly in hippocampus homogenates of aged and LPS-treated rats. The number of CA1 neurons decreased in aged rats. In the hippocampus of aged and LPS-treated rats astrocytes and microglia may help clearing apoptotic cellular debris possibly through CX3CL1 signalling. Our results indicate that astrocytes and microglia in the hippocampus of aged and LPS-infused rats possibly participate in the clearance of cellular debris associated with programmed cell death. The actions of astrocytes may represent either protective mechanisms to control inflammatory processes and the spread of further cellular damage to neighboring tissue, or they may contribute to neuronal damage in pathological conditions.
Conflict of interest statement
Figures


References
-
- Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60: 430–440. - PubMed
-
- Allen NJ, Barres BA (2009) Neuroscience: Glia - more than just brain glue. Nature 457: 675–677. - PubMed
-
- De Keyser J, Mostert JP, Koch MW (2008) Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci 267: 3–16. - PubMed
-
- Wenk GL, McGann K, Mencarelli A, Hauss-Wegrzyniak B, Del Soldato P, et al. (2000) Mechanisms to prevent the toxicity of chronic neuroinflammation on forebrain cholinergic neurons. Eur J Pharmacol 402: 77–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

