Use of postmortem human dura mater and scalp for deriving human fibroblast cultures
- PMID: 23028905
- PMCID: PMC3459947
- DOI: 10.1371/journal.pone.0045282
Use of postmortem human dura mater and scalp for deriving human fibroblast cultures
Abstract
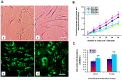
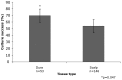
Fibroblasts can be collected from deceased individuals, grown in culture, reprogrammed into induced pluripotent stem cells (iPSCs), and then differentiated into a multitude of cell types, including neurons. Past studies have generated iPSCs from somatic cell biopsies from either animal or human subjects. Previously, fibroblasts have only been successfully cultured from postmortem human skin in two studies. Here we present data on fibroblast cell cultures generated from 146 scalp and/or 53 dura mater samples from 146 postmortem human brain donors. In our overall sample, the odds of successful dural culture was almost two-fold compared with scalp (OR = 1.95, 95% CI: [1.01, 3.9], p = 0.047). Using a paired design within subjects for whom both tissues were available for culture (n = 53), the odds of success for culture in dura was 16-fold as compared to scalp (OR = 16.0, 95% CI: [2.1-120.6], p = 0.0007). Unattended death, tissue donation source, longer postmortem interval (PMI), and higher body mass index (BMI) were associated with unsuccessful culture in scalp (all p<0.05), but not in dura. While scalp cells proliferated more and grew more rapidly than dura cells [F (1, 46) = 12.94, p<0.008], both tissues could be generated and maintained as fibroblast cell lines. Using a random sample of four cases, we found that both postmortem scalp and dura could be successfully reprogrammed into iPSC lines. Our study demonstrates that postmortem dura mater, and to a lesser extent, scalp, are viable sources of living fibroblasts for culture that can be used to generate iPSCs. These tissues may be accessible through existing brain tissue collections, which is critical for studying disorders such as neuropsychiatric diseases.
Conflict of interest statement
Figures

References
-
- Chamberlain SJ, Li XJ, Lalande M (2008) Induced pluripotent stem (iPS) cells as in vitro models of human neurogenetic disorders. Neurogenetics 9: 227–235. - PubMed
-
- Okada M, Oka M, Yoneda Y (2010) Effective culture conditions for the induction of pluripotent stem cells. Biochim Biophys Acta 1800: 956–963. - PubMed
-
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. - PubMed
-
- Meske V, Albert F, Wehser R, Ohm TG (1999) Culture of autopsy-derived fibroblasts as a tool to study systemic alterations in human neurodegenerative disorders such as Alzheimer’s disease–methodological investigations. J Neural Transm 106: 537–548. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

