Aquaporin expression contributes to human transurothelial permeability in vitro and is modulated by NaCl
- PMID: 23028946
- PMCID: PMC3454431
- DOI: 10.1371/journal.pone.0045339
Aquaporin expression contributes to human transurothelial permeability in vitro and is modulated by NaCl
Abstract
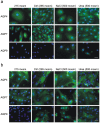
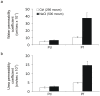
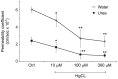
It is generally considered that the bladder is impervious and stores urine in unmodified form on account of the barrier imposed by the highly-specialised uro-epithelial lining. However, recent evidence, including demonstration of aquaporin (AQP) expression by human urothelium, suggests that urothelium may be able to modify urine content. Here we have we applied functional assays to an in vitro-differentiated normal human urothelial cell culture system and examined both whether AQP expression was responsive to changes in osmolality, and the effects of blocking AQP channels on water and urea transport. AQP3 expression was up-regulated by increased osmolality, but only in response to NaCl. A small but similar effect was seen with AQP9, but not AQP4 or AQP7. Differentiated urothelium revealed significant barrier function (mean TER 3862 Ω.cm(2)), with mean diffusive water and urea permeability coefficients of 6.33×10(-5) and 2.45×10(-5) cm/s, respectively. AQP blockade with mercuric chloride resulted in decreased water and urea flux. The diffusive permeability of urothelial cell sheets remained constant following conditioning in hyperosmotic NaCl, but there was a significant increase in water and urea flux across an osmotic gradient. Taken collectively with evidence emerging from studies in other species, our results support an active role for human urothelium in sensing and responding to hypertonic salt concentrations through alterations in AQP protein expression, with AQP channels providing a mechanism for modifying urine composition. These observations challenge the traditional concept of an impermeable bladder epithelium and suggest that the urothelium may play a modulatory role in water and salt homeostasis.
Conflict of interest statement
Figures



References
-
- Zeidel ML (1996) Low permeabilities of apical membranes of barrier epithelia: what makes watertight membranes watertight? Am J Physiol 271: F243–245. - PubMed
-
- Nelson RA, Jones JD, Wahner HW, McGill DB, Code CF (1975) Nitrogen metabolism in bears: urea metabolism in summer starvation and in winter sleep and role of urinary bladder in water and nitrogen conservation. Mayo Clin Proc 50: 141–146. - PubMed
-
- Hilson AJ, Lewis CA, Harland SJ (1990) The permeability of the human bladder to water assessed using tritiated water. Contrib Nephrol 79: 41–44. - PubMed
-
- Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML (1996) Permeability properties of the intact mammalian bladder epithelium. Am J Physiol 271: F886–894. - PubMed
-
- Rubenwolf PC, Georgopoulos NT, Clements LA, Feather S, Holland P, et al. (2009) Expression and localisation of aquaporin water channels in human urothelium in situ and in vitro. Eur Urol 56: 1013–1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

