5-aza-2'-deoxycytidine leads to reduced embryo implantation and reduced expression of DNA methyltransferases and essential endometrial genes
- PMID: 23028963
- PMCID: PMC3460940
- DOI: 10.1371/journal.pone.0045364
5-aza-2'-deoxycytidine leads to reduced embryo implantation and reduced expression of DNA methyltransferases and essential endometrial genes
Abstract
Background: The DNA demethylating agent 5-aza-2'-deoxycytidine (5-aza-CdR) incorporates into DNA and decreases DNA methylation, sparking interest in its use as a potential therapeutic agent. We aimed to determine the effects of maternal 5-aza-CdR treatment on embryo implantation in the mouse and to evaluate whether these effects are associated with decreased levels of DNA methyltransferases (Dnmts) and three genes (estrogen receptor α [Esr1], progesterone receptor [Pgr], and homeobox A10 [Hoxa10]) that are vital for control of endometrial changes during implantation.
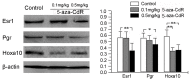
Methods and principal findings: Mice treated with 5-aza-CdR had a dose-dependent decrease in number of implantation sites, with defected endometrial decidualization and stromal cell proliferation. Western blot analysis on pseudo-pregnant day 3 (PD3) showed that 0.1 mg/kg 5-aza-CdR significantly repressed Dnmt3a protein level, and 0.5 mg/kg 5-aza-CdR significantly repressed Dnmt1, Dnmt3a, and Dnmt3b protein levels in the endometrium. On PD5, mice showed significantly decreased Dnmt3a protein level with 0.1 mg/kg 5-aza-CdR, and significantly decreased Dnmt1 and Dnmt3a with 0.5 mg/kg 5-aza-CdR. Immunohistochemical staining showed that 5-aza-CdR repressed DNMT expression in a cell type-specific fashion within the uterus, including decreased expression of Dnmt1 in luminal and/or glandular epithelium and of Dnmt3a and Dnmt3b in stroma. Furthermore, the 5' flanking regions of the Esr1, Pgr, and Hoxa10 were hypomethylated on PD5. Interestingly, the higher (0.5 mg/kg) dose of 5-aza-CdR decreased protein expression of Esr1, Pgr, and Hoxa10 in the endometrium on PD5 in both methylation-dependent and methylation-independent manners.
Conclusions: The effects of 5-aza-CdR on embryo implantation in mice were associated with altered expression of endometrial Dnmts and genes controlling endometrial changes, suggesting that altered gene methylation, and not cytotoxicity alone, contributes to implantation defects induced by 5-aza-CdR.
Conflict of interest statement
Figures





Similar articles
-
DNA Methylation Inhibitor 5-Aza-2'-Deoxycytidine Modulates Endometrial Receptivity Through Upregulating HOXA10 Expression.Reprod Sci. 2019 Jun;26(6):839-846. doi: 10.1177/1933719118815575. Epub 2018 Dec 6. Reprod Sci. 2019. PMID: 30522400
-
5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal.Mol Cell Biol. 2005 Jun;25(11):4727-41. doi: 10.1128/MCB.25.11.4727-4741.2005. Mol Cell Biol. 2005. Retraction in: Mol Cell Biol. 2022 May 19;42(5):e0054621. doi: 10.1128/mcb.00546-21. PMID: 15899874 Free PMC article. Retracted.
-
5-Aza-2'-deoxycytidine, a DNA methylation inhibitor, induces cytotoxicity, cell cycle dynamics and alters expression of DNA methyltransferase 1 and 3A in mouse hippocampus-derived neuronal HT22 cells.J Toxicol Environ Health A. 2017;80(22):1222-1229. doi: 10.1080/15287394.2017.1367143. Epub 2017 Sep 7. J Toxicol Environ Health A. 2017. PMID: 28880816
-
Antineoplastic activity of the DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine in anaplastic large cell lymphoma.Biochimie. 2012 Nov;94(11):2297-307. doi: 10.1016/j.biochi.2012.05.029. Epub 2012 Jun 9. Biochimie. 2012. PMID: 22687603 Free PMC article. Review.
-
Role of HOXA10 in pathologies of the endometrium.Rev Endocr Metab Disord. 2025 Feb;26(1):81-96. doi: 10.1007/s11154-024-09923-8. Epub 2024 Nov 5. Rev Endocr Metab Disord. 2025. PMID: 39499452 Review.
Cited by
-
Epigenetic modifications working in the decidualization and endometrial receptivity.Cell Mol Life Sci. 2020 Jun;77(11):2091-2101. doi: 10.1007/s00018-019-03395-9. Epub 2019 Dec 7. Cell Mol Life Sci. 2020. PMID: 31813015 Free PMC article. Review.
-
Tris(1,3-dichloro-2-propyl)phosphate Induces Genome-Wide Hypomethylation within Early Zebrafish Embryos.Environ Sci Technol. 2016 Sep 20;50(18):10255-63. doi: 10.1021/acs.est.6b03656. Epub 2016 Sep 9. Environ Sci Technol. 2016. PMID: 27574916 Free PMC article.
-
Aberrant DNA methyltransferase expression in pancreatic ductal adenocarcinoma development and progression.J Exp Clin Cancer Res. 2013 Nov 5;32(1):86. doi: 10.1186/1756-9966-32-86. J Exp Clin Cancer Res. 2013. PMID: 24423239 Free PMC article.
-
Epigenetic regulations through DNA methylation and hydroxymethylation: clues for early pregnancy in decidualization.Biomol Concepts. 2014 May;5(2):95-107. doi: 10.1515/bmc-2013-0036. Biomol Concepts. 2014. PMID: 25372745 Free PMC article. Review.
-
5-aza-2'-deoxycitidine inhibits cell proliferation, extracellular matrix formation and Wnt/β-catenin pathway in human uterine leiomyomas.Reprod Biol Endocrinol. 2021 Jul 8;19(1):106. doi: 10.1186/s12958-021-00790-5. Reprod Biol Endocrinol. 2021. PMID: 34233687 Free PMC article.
References
-
- Schmahl W, Torok P, Kriegel H (1984) Embryotoxicity of 5-azacytidine in mice. Phase- and dose-specificity studies. Arch Toxicol 55: 143–147. - PubMed
-
- Rosen MB, Chernoff N (2002) 5-Aza-2′-deoxycytidine-induced cytotoxicity and limb reduction defects in the mouse. Teratology 65: 180–190. - PubMed
-
- Cisneros FJ, Branch S (2003) 5-AZA-2′-deoxycytidine (5-AZA-CdR): a demethylating agent affecting development and reproductive capacity. J Appl Toxicol 23: 115–120. - PubMed
-
- Oka M, Meacham AM, Hamazaki T, Rodic N, Chang LJ, et al. (2005) De novo DNA methyltransferases Dnmt3a and Dnmt3b primarily mediate the cytotoxic effect of 5-aza-2′-deoxycytidine. Oncogene 24: 3091–3099. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

