VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation
- PMID: 23029000
- PMCID: PMC3448636
- DOI: 10.1371/journal.pone.0045425
VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation
Abstract
Background: Signals generated by the inflammed intestine are thought to contribute to metabolic derangement. The intestinal microbiota contributes to instructing the immune system beyond the intestinal wall and its modulation is a potential target for treating systemic disorders.
Aims: To investigate the pathogenetic role of low grade intestinal inflammation in the development of steatohepatitis and atherosclerosis in a model of genetic dyslipidemia and to test the therapeutic potential of a probiotics intervention in protecting against development of these disorders.
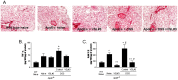
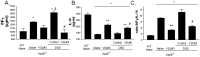
Results: ApoE(-/-) mice were randomized to receive vehicle or VSL#3, a mixture of eight probiotics, at the dose of 20×10(9) colony-forming units/kg/day for three months alone or in combination with 0.2% of dextran sulfate sodium (DSS) in drinking water. Administering DSS to ApoE(-/-) mice failed to induce signs and symptoms of colitis but increased intestinal permeability to dextran FITC and, while had no effect on serum lipids, increased the blood levels of markers of liver injury and insulin resistance. DSS administration associated with low level inflammation of intestinal and mesenteric adipose tissues, caused liver histopathology features of steatohepatitis and severe atherosclerotic lesions in the aorta. These changes were prevented by VSL#3 intervention. Specifically, VSL#3 reversed insulin resistance, prevented development of histologic features of mesenteric adipose tissue inflammation, steatohepatitis and reduced the extent of aortic plaques. Conditioned media obtained from cultured probiotics caused the direct transactivation of peroxisome proliferator-activated receptor-γ, Farnesoid-X-receptors and vitamin D receptor.
Conclusions: Low grade intestinal inflammation drives a transition from steatosis to steatohepatitis and worsens the severity of atherosclerosis in a genetic model of dyslipidemia. VSL#3 intervention modulates the expression of nuclear receptors, corrects for insulin resistance in liver and adipose tissues and protects against development of steatohepatitis and atherosclerosis.
Conflict of interest statement
Figures





References
-
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645. - PubMed
-
- Reinhard W, Holmer SR, Fischer M, Gloeckner C, Hubauer U, et al. (2006) Association of the metabolic syndrome with early coronary disease in families with frequent myocardial infarction. Am J Cardiol 97: 964–967. - PubMed
-
- Loria P, Lonardo A, Carulli N (2005) Should nonalcoholic fatty liver disease be renamed? Dig Dis 23: 72–82. - PubMed
-
- Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H (2007) Liver fat in the metabolic syndrome. J Clin Endocrinol Metab 92: 3490–3497. - PubMed
-
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, et al. (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917–923. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

