Characterization of PTPRG in knockdown and phosphatase-inactive mutant mice and substrate trapping analysis of PTPRG in mammalian cells
- PMID: 23029056
- PMCID: PMC3447766
- DOI: 10.1371/journal.pone.0045500
Characterization of PTPRG in knockdown and phosphatase-inactive mutant mice and substrate trapping analysis of PTPRG in mammalian cells
Abstract
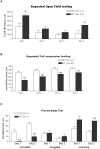
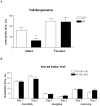
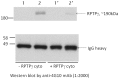
Receptor tyrosine phosphatase gamma (PTPRG, or RPTPγ) is a mammalian receptor-like tyrosine phosphatase which is highly expressed in the nervous system as well as other tissues. Its function and biochemical characteristics remain largely unknown. We created a knockdown (KD) line of this gene in mouse by retroviral insertion that led to 98-99% reduction of RPTPγ gene expression. The knockdown mice displayed antidepressive-like behaviors in the tail-suspension test, confirming observations by Lamprianou et al. 2006. We investigated this phenotype in detail using multiple behavioral assays. To see if the antidepressive-like phenotype was due to the loss of phosphatase activity, we made a knock-in (KI) mouse in which a mutant, RPTPγ C1060S, replaced the wild type. We showed that human wild type RPTPγ protein, expressed and purified, demonstrated tyrosine phosphatase activity, and that the RPTPγ C1060S mutant was completely inactive. Phenotypic analysis showed that the KI mice also displayed some antidepressive-like phenotype. These results lead to a hypothesis that an RPTPγ inhibitor could be a potential treatment for human depressive disorders. In an effort to identify a natural substrate of RPTPγ for use in an assay for identifying inhibitors, "substrate trapping" mutants (C1060S, or D1028A) were studied in binding assays. Expressed in HEK293 cells, these mutant RPTPγs retained a phosphorylated tyrosine residue, whereas similarly expressed wild type RPTPγ did not. This suggested that wild type RPTPγ might auto-dephosphorylate which was confirmed by an in vitro dephosphorylation experiment. Using truncation and mutagenesis studies, we mapped the auto-dephosphorylation to the Y1307 residue in the D2 domain. This novel discovery provides a potential natural substrate peptide for drug screening assays, and also reveals a potential functional regulatory site for RPTPγ. Additional investigation of RPTPγ activity and regulation may lead to a better understanding of the biochemical underpinnings of human depression.
Conflict of interest statement
Figures





References
-
- Wang J, Bixby JL (1999) Receptor tyrosine phosphatase-delta is a homophilic, neurite-promoting cell adhesion molecular for CNS neurons. Mol Cell Neurosci 14: 370–384. - PubMed
-
- Johnson KG, van Vactor D (2003) Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev 83: 1–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

