Annexin A10 in human oral cancer: biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways
- PMID: 23029062
- PMCID: PMC3444476
- DOI: 10.1371/journal.pone.0045510
Annexin A10 in human oral cancer: biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways
Abstract
Background: Annexins are calcium and phospholipid binding proteins that form an evolutionary conserved multigene family. Considerable evidence indicates that annexin A10 (ANXA10) is involved in tumoral progression, although little is known about its role in human oral carcinogenesis. In this study, we investigated the involvement of ANXA10 in oral squamous cell carcinoma (OSCC).
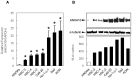
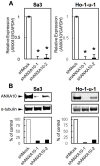
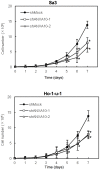
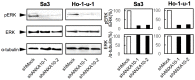
Methodology/principal findings: ANXA10 mRNA and protein expressions were assessed by quantitative reverse transcriptase polymerase chain reaction and immunoblotting, and we conducted a proliferation assay and cell-cycle analysis in ANXA10 knockdown cells in vitro. We evaluated the correlation between the ANXA10 expression status in 100 primary OSCCs and the clinicopathological features by immunohistochemistry. ANXA10 mRNA and protein expression levels were up-regulated in all cellular lines examined (n = 7, p<0.05). ANXA10 knockdown cells showed that cellular proliferation decreased by inactivation of extracellular regulated kinase (ERK) (p<0.05), and cell-cycle arrest at the G1 phase resulted from up-regulation of cyclin-dependent kinase inhibitors. ANXA10 protein expression in primary OSCCs was also significantly greater than in normal counterparts (p<0.05), and higher expression was correlated with tumoral size (p = 0.027).
Conclusions/significance: Our results proposed for the first time that ANXA10 is an indicator of cellular proliferation in OSCCs. Our results suggested that ANXA10 expression might indicate cellular proliferation and ANXA10 might be a potential therapeutic target for the development of new treatments for OSCCs.
Conflict of interest statement
Figures

References
-
- Chang KS, Wang G, Freireich EJ, Daly M, Naylor SL, et al. (1992) Specific expression of the annexin VIII gene in acute promyelocytic leukemia. Blood 79: 1802–10. - PubMed
-
- Pencil SD, Toth M (1998) Elevated levels of annexin I protein in vitro and in vivo in rat and human mammary adenocarcinoma. Clin Exp Metastasis 16: 113–21. - PubMed
-
- Sarkar A, Yang P, Fan YH, Mu ZM, Hauptmann R, et al. (1994) Regulation of the expression of annexin VIII in acute promyelocytic leukemia. Blood 84: 279–86. - PubMed
-
- Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimura T, et al. (1996) Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology 24: 72–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

