Myogenic potential of whole bone marrow mesenchymal stem cells in vitro and in vivo for usage in urinary incontinence
- PMID: 23029081
- PMCID: PMC3448658
- DOI: 10.1371/journal.pone.0045538
Myogenic potential of whole bone marrow mesenchymal stem cells in vitro and in vivo for usage in urinary incontinence
Abstract
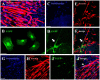
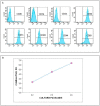
Urinary incontinence, defined as the complaint of any involuntary loss of urine, is a pathological condition, which affects 30% females and 15% males over 60, often following a progressive decrease of rhabdosphincter cells due to increasing age or secondary to damage to the pelvic floor musculature, connective tissue and/or nerves. Recently, stem cell therapy has been proposed as a source for cell replacement and for trophic support to the sphincter. To develop new therapeutic strategies for urinary incontinence, we studied the interaction between mesenchymal stem cells (MSCs) and muscle cells in vitro; thereafter, aiming at a clinical usage, we analyzed the supporting role of MSCs for muscle cells in vitro and in in vivo xenotransplantation. MSCs can express markers of the myogenic cell lineages and give rise, under specific cell culture conditions, to myotube-like structures. Nevertheless, we failed to obtain mixed myotubes both in vitro and in vivo. For in vivo transplantation, we tested a new protocol to collect human MSCs from whole bone marrow, to get larger numbers of cells. MSCs, when transplanted into the pelvic muscles close to the external urethral sphincter, survived for a long time in absence of immunosuppression, and migrated into the muscle among fibers, and towards neuromuscular endplates. Moreover, they showed low levels of cycling cells, and did not infiltrate blood vessels. We never observed formation of cell masses suggestive of tumorigenesis. Those which remained close to the injection site showed an immature phenotype, whereas those in the muscle had more elongated morphologies. Therefore, MSCs are safe and can be easily transplanted without risk of side effects in the pelvic muscles. Further studies are needed to elucidate their integration into muscle fibers, and to promote their muscular transdifferentiation either before or after transplantation.
Conflict of interest statement
Figures



References
-
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, et al. (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 21: 5–26. - PubMed
-
- Strasser H, Tiefenthaler M, Steinlechner M, Eder I, Bartsch G, et al. (2000) Age dependent apoptosis and loss of rhabdosphincter cells. The Journal of Urology 164: 1781–1785. - PubMed
-
- Nikolavsky D, Chancellor MB (2010) Stem cell therapy for stress urinary incontinence. Neurourol Urodyn 29 Suppl 1S36–41. - PubMed
-
- Corcos J, Loutochin O, Campeau L, Eliopoulos N, Bouchentouf M, et al. (2010) Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourology and urodynamics 30: 447–455. - PubMed
-
- Sweat SD, Lightner DJ (1999) Complications of sterile abscess formation and pulmonary embolismfollowing periurethral bulking agents. J Urol 161: 93–96. - PubMed

