Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2
- PMID: 23029099
- PMCID: PMC3447807
- DOI: 10.1371/journal.pone.0045562
Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2
Abstract
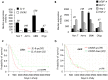
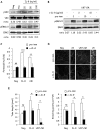
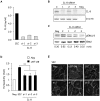
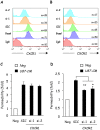
Glioblastoma constitutes the most aggressive and deadly of brain tumors. As yet, both conventional and molecular-based therapies have met with limited success in treatment of this cancer. Among other explanations, the heterogeneity of glioblastoma and the associated microenvironment contribute to its development, as well as resistance and recurrence in response to treatments. Increased vascularity suggests that tumor angiogenesis plays an important role in glioblastoma progression. However, the molecular crosstalk between endothelial and glioblastoma cells requires further investigation. To examine the effects of glioblastoma-derived signals on endothelial homeostasis, glioblastoma cell secretions were collected and used to treat brain endothelial cells. Here, we present evidence that the glioblastoma secretome provides pro-angiogenic signals sufficient to disrupt VE-cadherin-mediated cell-cell junctions and promote endothelial permeability in brain microvascular endothelial cells. An unbiased angiogenesis-specific antibody array screen identified the chemokine, interleukin-8, which was further demonstrated to function as a key factor involved in glioblastoma-induced permeability, mediated through its receptor CXCR2 on brain endothelia. This underappreciated interface between glioblastoma cells and associated endothelium may inspire the development of novel therapeutic strategies to induce tumor regression by preventing vascular permeability and inhibiting angiogenesis.
Conflict of interest statement
Figures


References
-
- Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, et al. (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8: 610–622. - PubMed
-
- Plate KH, Breier G, Weich HA, Mennel HD, Risau W (1994) Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer 59: 520–529. - PubMed
-
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, et al. (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11: 69–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

