A lateral flow assay for quantitative detection of amplified HIV-1 RNA
- PMID: 23029134
- PMCID: PMC3448666
- DOI: 10.1371/journal.pone.0045611
A lateral flow assay for quantitative detection of amplified HIV-1 RNA
Abstract
Although the accessibility of HIV treatment in developing nations has increased dramatically over the past decade, viral load testing to monitor the response of patients receiving therapy is often unavailable. Existing viral load technologies are often too expensive or resource-intensive for poor settings, and there is no appropriate HIV viral load test currently available at the point-of-care in low resource settings. Here, we present a lateral flow assay that employs gold nanoparticle probes and gold enhancement solution to detect amplified HIV RNA quantitatively. Preliminary results show that, when coupled with nucleic acid sequence based amplification (NASBA), this assay can detect concentrations of HIV RNA that match the clinically relevant range of viral loads found in HIV patients. The lateral flow test is inexpensive, simple and rapid to perform, and requires few resources. Our results suggest that the lateral flow assay may be integrated with amplification and sample preparation technologies to serve as an HIV viral load test for low-resource settings.
Conflict of interest statement
Figures
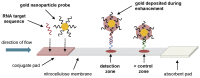
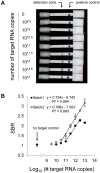
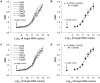
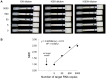
Similar articles
-
Clinical comparison of branched DNA and reverse transcriptase-PCR and nucleic acid sequence-based amplification assay for the quantitation of circulating recombinant form_BC HIV-1 RNA in plasma.AIDS. 2007 Dec;21 Suppl 8:S27-32. doi: 10.1097/01.aids.0000304693.16767.66. AIDS. 2007. PMID: 18172387
-
Isothermal Amplification with a Target-Mimicking Internal Control and Quantitative Lateral Flow Readout for Rapid HIV Viral Load Testing in Low-Resource Settings.Anal Chem. 2022 Jan 18;94(2):1011-1021. doi: 10.1021/acs.analchem.1c03960. Epub 2021 Dec 17. Anal Chem. 2022. PMID: 34920665 Free PMC article.
-
Amplicor HIV monitor, NASBA HIV-1 RNA QT and quantiplex HIV RNA version 2.0 viral load assays: a Canadian evaluation.J Clin Virol. 1998 Dec;11(3):189-202. doi: 10.1016/s0928-0197(98)00058-0. J Clin Virol. 1998. PMID: 9949955
-
Laboratory operations, specimen processing, and handling for viral load testing and surveillance.J Infect Dis. 2010 Apr 15;201 Suppl 1:S27-36. doi: 10.1086/650390. J Infect Dis. 2010. PMID: 20225943 Review.
-
Measurement of HIV-1 viral load for drug resistance surveillance using dried blood spots: literature review and modeling of contribution of DNA and RNA.AIDS Rev. 2014 Jul-Sep;16(3):160-71. AIDS Rev. 2014. PMID: 25221990 Review.
Cited by
-
Advanced trap lateral flow immunoassay sensor for the detection of cortisol in human bodily fluids.Sci Rep. 2021 Nov 19;11(1):22580. doi: 10.1038/s41598-021-02084-7. Sci Rep. 2021. PMID: 34799635 Free PMC article.
-
Moving toward rapid and low-cost point-of-care molecular diagnostics with a repurposed 3D printer and RPA.Anal Biochem. 2018 Mar 15;545:4-12. doi: 10.1016/j.ab.2018.01.008. Epub 2018 Jan 12. Anal Biochem. 2018. PMID: 29339059 Free PMC article.
-
The Emergency Medical Service Microbiome.Appl Environ Microbiol. 2018 Feb 14;84(5):e02098-17. doi: 10.1128/AEM.02098-17. Print 2018 Mar 1. Appl Environ Microbiol. 2018. PMID: 29222105 Free PMC article. Review.
-
Multivalent nanoparticle networks enable point-of-care detection of human phospholipase-A2 in serum.ACS Nano. 2015 Mar 24;9(3):2565-2573. doi: 10.1021/nn5057595. Epub 2015 Mar 10. ACS Nano. 2015. PMID: 25756526 Free PMC article.
-
Rapid Point-of-Care Testing for Detection of HIV and Clinical Monitoring.ISRN AIDS. 2013 May 23;2013:287269. doi: 10.1155/2013/287269. ISRN AIDS. 2013. PMID: 24052887 Free PMC article. Review.
References
-
- (2010) Global report: UNAIDS report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS.
-
- (2009) Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. World Health Organization.
-
- Volberding PA, Deeks SG (2010) Antiretroviral therapy and management of HIV infection. Lancet 376: 49–62. - PubMed
-
- Moore D, Awor A, Downing R, Kaplan J, Montaner J, et al. (2008) CD4(+) T-Cell Count Monitoring Does Not Accurately Identify HIV-Infected Adults With Virologic Failure Receiving Antiretroviral Therapy. Jaids-Journal of Acquired Immune Deficiency Syndromes 49: 477–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

