NaCl potentiates human fibrocyte differentiation
- PMID: 23029177
- PMCID: PMC3445484
- DOI: 10.1371/journal.pone.0045674
NaCl potentiates human fibrocyte differentiation
Abstract
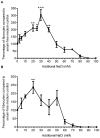
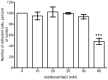
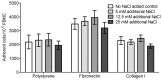
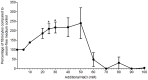
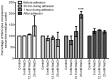
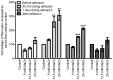
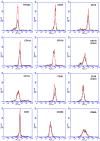
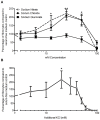
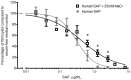
Excessive NaCl intake is associated with a variety of fibrosing diseases such as renal and cardiac fibrosis. This association has been attributed to increased blood pressure as the result of high NaCl intake. However, studies in patients with high NaCl intake and fibrosis reveal a connection between NaCl intake and fibrosis that is independent of blood pressure. We find that increasing the extracellular concentration of NaCl to levels that may occur in human blood after high-salt intake can potentiate, in serum-free culture conditions, the differentiation of freshly-isolated human monocytes into fibroblast-like cells called fibrocytes. NaCl affects the monocytes directly during their adhesion. Potassium chloride and sodium nitrate also potentiate fibrocyte differentiation. The plasma protein Serum Amyloid P (SAP) inhibits fibrocyte differentiation. High levels of extracellular NaCl change the SAP Hill coefficient from 1.7 to 0.8, and cause a four-fold increase in the concentration of SAP needed to inhibit fibrocyte differentiation by 95%. Together, our data suggest that NaCl potentiates fibrocyte differentiation. NaCl-increased fibrocyte differentiation may thus contribute to NaCl-increased renal and cardiac fibrosis.
Conflict of interest statement
Figures

References
-
- Moeller A, Gilpin SE, Ask K, Cox G, Cook D, et al. (2009) Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 179: 588–594. - PubMed
-
- McAnulty RJ (2007) Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol 39: 666–671. - PubMed
-
- Quan TE, Cowper SE, Bucala R (2006) The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep 8: 145–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

