Role of vegetation-associated protease activity in valve destruction in human infective endocarditis
- PMID: 23029186
- PMCID: PMC3447824
- DOI: 10.1371/journal.pone.0045695
Role of vegetation-associated protease activity in valve destruction in human infective endocarditis
Abstract
Aims: Infective endocarditis (IE) is characterized by septic thrombi (vegetations) attached on heart valves, consisting of microbial colonization of the valvular endocardium, that may eventually lead to congestive heart failure or stroke subsequent to systemic embolism. We hypothesized that host defense activation may be directly involved in tissue proteolytic aggression, in addition to pathogenic effects of bacterial colonization.
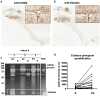
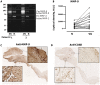
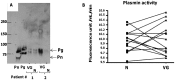
Methods and results: IE valve samples collected during surgery (n = 39) were dissected macroscopically by separating vegetations (VG) and the surrounding damaged part of the valve from the adjacent, apparently normal (N) valvular tissue. Corresponding conditioned media were prepared separately by incubation in culture medium. Histological analysis showed an accumulation of platelets and polymorphonuclear neutrophils (PMNs) at the interface between the VG and the underlying tissue. Apoptotic cells (PMNs and valvular cells) were abundantly detected in this area. Plasminogen activators (PA), including urokinase (uPA) and tissue (tPA) types were also associated with the VG. Secreted matrix metalloproteinase (MMP) 9 was also increased in VG, as was leukocyte elastase and myeloperoxidase (MPO). The presence of neutrophil extracellular traps (NETs) associating MPO and externalized nucleosomes, was shown by immunostaining in the VG. Both MPO and cell-free DNA were released in larger amounts by VG than N samples, suggesting bacterial activation of PMNs within the vegetation. Finally, evidence of proteolytic tissue damage was obtained by the release of fragments of extracellular matrix components such as fibrinogen and fibronectin, as well as protease-sensitive receptors such as the uPA receptor.
Conclusion: Our data obtained using human IE valves suggest that septic vegetations represent an important source of proteases originating from massive leukocyte recruitment and activation of the host plasminergic system. The latter forms a potential therapeutic target to minimize valvular tissue degradation independently from that induced by bacterial proteases.
Conflict of interest statement
Figures




References
-
- Delahaye F, Goulet V, Lacassin F, Ecochard R, Selton-Suty C, et al. (1995) Characteristics of infective endocarditis in France in 1991. A 1-year survey. Eur Heart J 16: 394–401. - PubMed
-
- Hoen B, Alla F, Selton-Suty C, Beguinot I, Bouvet A, et al. (2002) Changing profile of infective endocarditis: results of a 1-year survey in France. Jama 288: 75–81. - PubMed
-
- Moreillon P, Que YA (2004) Infective endocarditis. Lancet 363: 139–149. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

