Mitochondrial fusion is essential for steroid biosynthesis
- PMID: 23029265
- PMCID: PMC3448708
- DOI: 10.1371/journal.pone.0045829
Mitochondrial fusion is essential for steroid biosynthesis
Abstract
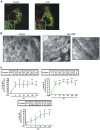
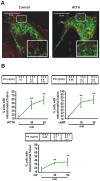
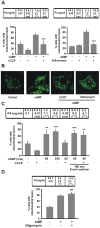
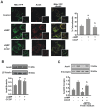
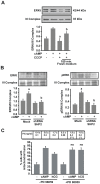
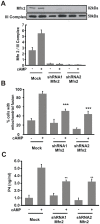
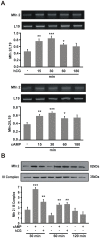
Although the contribution of mitochondrial dynamics (a balance in fusion/fission events and changes in mitochondria subcellular distribution) to key biological process has been reported, the contribution of changes in mitochondrial fusion to achieve efficient steroid production has never been explored. The mitochondria are central during steroid synthesis and different enzymes are localized between the mitochondria and the endoplasmic reticulum to produce the final steroid hormone, thus suggesting that mitochondrial fusion might be relevant for this process. In the present study, we showed that the hormonal stimulation triggers mitochondrial fusion into tubular-shaped structures and we demonstrated that mitochondrial fusion does not only correlate-with but also is an essential step of steroid production, being both events depend on PKA activity. We also demonstrated that the hormone-stimulated relocalization of ERK1/2 in the mitochondrion, a critical step during steroidogenesis, depends on mitochondrial fusion. Additionally, we showed that the SHP2 phosphatase, which is required for full steroidogenesis, simultaneously modulates mitochondrial fusion and ERK1/2 localization in the mitochondrion. Strikingly, we found that mitofusin 2 (Mfn2) expression, a central protein for mitochondrial fusion, is upregulated immediately after hormone stimulation. Moreover, Mfn2 knockdown is sufficient to impair steroid biosynthesis. Together, our findings unveil an essential role for mitochondrial fusion during steroidogenesis. These discoveries highlight the importance of organelles' reorganization in specialized cells, prompting the exploration of the impact that organelle dynamics has on biological processes that include, but are not limited to, steroid synthesis.
Conflict of interest statement
Figures

Similar articles
-
Mitochondrial fusion and ERK activity regulate steroidogenic acute regulatory protein localization in mitochondria.PLoS One. 2014 Jun 19;9(6):e100387. doi: 10.1371/journal.pone.0100387. eCollection 2014. PLoS One. 2014. PMID: 24945345 Free PMC article.
-
Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling.J Endocrinol. 2007 Apr;193(1):53-63. doi: 10.1677/JOE-06-0201. J Endocrinol. 2007. PMID: 17400803
-
Angiotensin II stimulation promotes mitochondrial fusion as a novel mechanism involved in protein kinase compartmentalization and cholesterol transport in human adrenocortical cells.J Steroid Biochem Mol Biol. 2019 Sep;192:105413. doi: 10.1016/j.jsbmb.2019.105413. Epub 2019 Jun 13. J Steroid Biochem Mol Biol. 2019. PMID: 31202858
-
The spatial and temporal regulation of the hormonal signal. Role of mitochondria in the formation of a protein complex required for the activation of cholesterol transport and steroids synthesis.Mol Cell Endocrinol. 2013 May 22;371(1-2):26-33. doi: 10.1016/j.mce.2012.12.024. Epub 2013 Jan 26. Mol Cell Endocrinol. 2013. PMID: 23357790 Review.
-
Shaping mitochondrial dynamics: The role of cAMP signalling.Biochem Biophys Res Commun. 2018 May 27;500(1):65-74. doi: 10.1016/j.bbrc.2017.05.041. Epub 2017 May 10. Biochem Biophys Res Commun. 2018. PMID: 28501614 Review.
Cited by
-
Regulation of adrenocortical steroid hormone production by RhoA-diaphanous 1 signaling and the cytoskeleton.Mol Cell Endocrinol. 2013 May 22;371(1-2):79-86. doi: 10.1016/j.mce.2012.11.014. Epub 2012 Nov 24. Mol Cell Endocrinol. 2013. PMID: 23186810 Free PMC article. Review.
-
Growing Up Under Constant Light: A Challenge to the Endocrine Function of the Leydig Cells.Front Endocrinol (Lausanne). 2021 Mar 16;12:653602. doi: 10.3389/fendo.2021.653602. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33796081 Free PMC article.
-
miR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4.BMC Genet. 2020 Mar 14;21(1):33. doi: 10.1186/s12863-020-0836-7. BMC Genet. 2020. PMID: 32171241 Free PMC article.
-
Mitochondrial Dynamics as Potential Modulators of Hormonal Therapy Effectiveness in Males.Biology (Basel). 2023 Apr 3;12(4):547. doi: 10.3390/biology12040547. Biology (Basel). 2023. PMID: 37106748 Free PMC article. Review.
-
CRISPR/Cas9‒Mediated Tspo Gene Mutations Lead to Reduced Mitochondrial Membrane Potential and Steroid Formation in MA-10 Mouse Tumor Leydig Cells.Endocrinology. 2018 Feb 1;159(2):1130-1146. doi: 10.1210/en.2017-03065. Endocrinology. 2018. PMID: 29300865 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

