Interaction between lysophosphatidic acid, prostaglandins and the endocannabinoid system during the window of implantation in the rat uterus
- PMID: 23029388
- PMCID: PMC3460956
- DOI: 10.1371/journal.pone.0046059
Interaction between lysophosphatidic acid, prostaglandins and the endocannabinoid system during the window of implantation in the rat uterus
Abstract
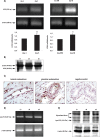
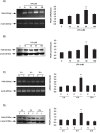
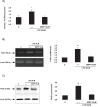
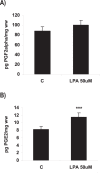
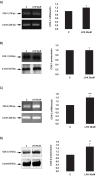
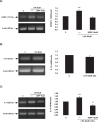
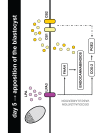
Bioactive lipid molecules as lysophosphatidic acid (LPA), prostaglandins (PG) and endocannabinoids are important mediators of embryo implantation. Based on previous published data we became interested in studying the interaction between these three groups of lipid derivatives in the rat uterus during the window of implantation. Thus, we adopted a pharmacological approach in vitro using LPA, DGPP (a selective antagonist of LPA3, an LPA receptor), endocannabinoids' receptor selective antagonists (AM251 and AM630) and non selective (indomethacin) and selective (NS-398) inhibitors of cyclooxygenase-1 and 2 enzymes. Cyclooxygenase isoforms participate in prostaglandins' synthesis. The incubation of the uterus from rats pregnant on day 5 of gestation (implantation window) with LPA augmented the activity and the expression of fatty acid amide hydrolase, the main enzyme involved in the degradation of endocannabinoids in the rodent uteri, suggesting that LPA decreased endocannabinoids' levels during embryo implantation. It has been reported that high endocannabinoids are deleterious for implantation. Also, LPA increased PGE2 production and cyclooxygenase-2 expression. The incubation of LPA with indomethacin or NS-398 reversed the increment in PGE2 production, suggesting that cyclooxygenase-2 was the isoform involved in LPA effect. PGs are important mediators of decidualization and vascularization at the implantation sites. All these effects were mediated by LPA3, as the incubation with DGPP completely reversed LPA stimulatory actions. Besides, we also observed that endocannabinoids mediated the stimulatory effect of LPA on cyclooxygenase-2 derived PGE2 production, as the incubation of LPA with AM251 or AM630 completely reversed LPA effect. Also, LPA augmented via LPA3 decidualization and vascularization markers. Overall, the results presented here demonstrate the participation of LPA3 in the process of implantation through the interaction with other groups of lipid molecules, prostaglandins and endocannabinoids, which prepare the uterine milieu for embryo invasion during the window of implantation.
Conflict of interest statement
Figures


References
-
- Dey SK (2005) Fatty link to fertility. Nature 435: 34–35. - PubMed
-
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, et al. (1997) Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91: 197–208. - PubMed
-
- Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, et al. (1997) Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390: 622–625. - PubMed
-
- Lim H, Song H, Paria BC, Reese J, Das SK, et al. (2002) Molecules in blastocyst implantation: uterine and embryonic perspectives. Vitam Horm 64: 43–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

