Postconditioning with inhaled carbon monoxide counteracts apoptosis and neuroinflammation in the ischemic rat retina
- PMID: 23029526
- PMCID: PMC3460901
- DOI: 10.1371/journal.pone.0046479
Postconditioning with inhaled carbon monoxide counteracts apoptosis and neuroinflammation in the ischemic rat retina
Abstract
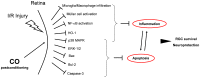
Purpose: Ischemia and reperfusion injury (I/R) of neuronal structures and organs is associated with increased morbidity and mortality due to neuronal cell death. We hypothesized that inhalation of carbon monoxide (CO) after I/R injury ('postconditioning') would protect retinal ganglion cells (RGC).
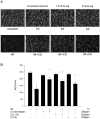
Methods: Retinal I/R injury was performed in Sprague-Dawley rats (n = 8) by increasing ocular pressure (120 mmHg, 1 h). Rats inhaled room air or CO (250 ppm) for 1 h immediately following ischemia or with 1.5 and 3 h latency. Retinal tissue was harvested to analyze Bcl-2, Bax, Caspase-3, HO-1 expression and phosphorylation of the nuclear transcription factor (NF)-κB, p38 and ERK-1/2 MAPK. NF-κB activation was determined and inhibition of ERK-1/2 was performed using PD98059 (2 mg/kg). Densities of fluorogold prelabeled RGC were analyzed 7 days after injury. Microglia, macrophage and Müller cell activation and proliferation were evaluated by Iba-1, GFAP and Ki-67 staining.
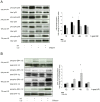
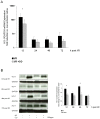
Results: Inhalation of CO after I/R inhibited Bax and Caspase-3 expression (Bax: 1.9 ± 0.3 vs. 1.4 ± 0.2, p = 0.028; caspase-3: 2.0 ± 0.2 vs. 1.5 ± 0.1, p = 0.007; mean ± S.D., fold induction at 12 h), while expression of Bcl-2 was induced (1.2 ± 0.2 vs. 1.6 ± 0.2, p = 0.001; mean ± S.D., fold induction at 12 h). CO postconditioning suppressed retinal p38 phosphorylation (p = 0.023 at 24 h) and induced the phosphorylation of ERK-1/2 (p<0.001 at 24 h). CO postconditioning inhibited the expression of HO-1. The activation of NF-κB, microglia and Müller cells was potently inhibited by CO as well as immigration of proliferative microglia and macrophages into the retina. CO protected I/R-injured RGC with a therapeutic window at least up to 3 h (n = 8; RGC/mm(2); mean ± S.D.: 1255 ± 327 I/R only vs. 1956 ± 157 immediate CO treatment, vs. 1830 ± 109 1.5 h time lag and vs. 1626 ± 122 3 h time lag; p<0.001). Inhibition of ERK-1/2 did not counteract the CO effects (RGC/mm(2): 1956 ± 157 vs. 1931 ± 124, mean ± S.D., p = 0.799).
Conclusion: Inhaled CO, administered after retinal ischemic injury, protects RGC through its strong anti-apoptotic and anti-inflammatory effects.
Conflict of interest statement
Figures






References
-
- Wong GY, Warner DO, Schroeder DR, Offord KP, Warner MA, et al. (2000) Risk of surgery and anesthesia for ischemic stroke. Anesthesiology 92: 425–432. - PubMed
-
- Mashour GA, Shanks AM, Kheterpal S (2011) Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology 114: 1289–1296. - PubMed
-
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, et al. (2006) 1,026 experimental treatments in acute stroke. Ann Neurol 59: 467–477. - PubMed
-
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, et al. (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428. - PubMed
-
- Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, et al. (2003) Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem 278: 1248–1258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

