Inhibition of fatty acid metabolism reduces human myeloma cells proliferation
- PMID: 23029529
- PMCID: PMC3460894
- DOI: 10.1371/journal.pone.0046484
Inhibition of fatty acid metabolism reduces human myeloma cells proliferation
Abstract
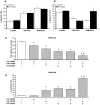
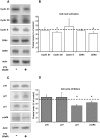
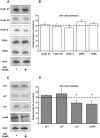
Multiple myeloma is a haematological malignancy characterized by the clonal proliferation of plasma cells. It has been proposed that targeting cancer cell metabolism would provide a new selective anticancer therapeutic strategy. In this work, we tested the hypothesis that inhibition of β-oxidation and de novo fatty acid synthesis would reduce cell proliferation in human myeloma cells. We evaluated the effect of etomoxir and orlistat on fatty acid metabolism, glucose metabolism, cell cycle distribution, proliferation, cell death and expression of G1/S phase regulatory proteins in myeloma cells. Etomoxir and orlistat inhibited β-oxidation and de novo fatty acid synthesis respectively in myeloma cells, without altering significantly glucose metabolism. These effects were associated with reduced cell viability and cell cycle arrest in G0/G1. Specifically, etomoxir and orlistat reduced by 40-70% myeloma cells proliferation. The combination of etomoxir and orlistat resulted in an additive inhibitory effect on cell proliferation. Orlistat induced apoptosis and sensitized RPMI-8226 cells to apoptosis induction by bortezomib, whereas apoptosis was not altered by etomoxir. Finally, the inhibitory effect of both drugs on cell proliferation was associated with reduced p21 protein levels and phosphorylation levels of retinoblastoma protein. In conclusion, inhibition of fatty acid metabolism represents a potential therapeutic approach to treat human multiple myeloma.
Conflict of interest statement
Figures



References
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7: 11–20. - PubMed
-
- Warburg O (1956) On the origin of cancer cells. Science 123: 309–314. - PubMed
-
- Warburg O (1956) On respiratory impairment in cancer cells. Science 124: 269–270. - PubMed
-
- Ortega AD, Sanchez-Arago M, Giner-Sanchez D, Sanchez-Cenizo L, Willers I, et al. (2009) Glucose avidity of carcinomas. Cancer Lett 276: 125–135. - PubMed
-
- Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7: 763–777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

