Mechanistic investigation of the nickel-catalyzed Suzuki reaction of N,O-acetals: evidence for boronic acid assisted oxidative addition and an iminium activation pathway
- PMID: 23030789
- PMCID: PMC4807852
- DOI: 10.1021/ja3079362
Mechanistic investigation of the nickel-catalyzed Suzuki reaction of N,O-acetals: evidence for boronic acid assisted oxidative addition and an iminium activation pathway
Abstract
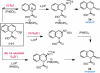
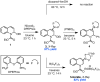
The mechanism of a recently reported Suzuki coupling reaction of quinoline-derived allylic N,O-acetals has been studied using a combination of structural, stereochemical, and kinetic isotope effect experiments. The data indicate that C-O activation is facilitated by Lewis acid assistance from the boronic acid coupling partner and an ionic S(N)1-like mechanism accounts for oxidative addition. In this context, we demonstrate the first direct observation of oxidative addition to a quinolinium salt. Notably, this mechanism is distinct from the more commonly described S(N)2(')-type oxidative addition of low-valent transition metals to most allylic electrophiles.
Figures


References
-
-
For examples see: Trost BM, Verhoeven TR. J. Am. Chem. Soc. 1980;102:4730. Hayashi T, Hagihara T, Konishi M, Kumada M. J. Am. Chem. Soc. 1983;105:7767. Sheffy FK, Godschalx JP, Stille JK. J. Am. Chem. Soc. 1984;106:4833. Kurosawa H, Kajimaru H, Ogoshi S, Yoneda H, Miki K, Kasai N, Murai S, Ikeda I. J. Am. Chem. Soc. 1992;114:8417. In this paper, we designate stereospecific allylic oxidative addition that proceeds with inversion as an SN2(′) mechanism.
-
-
-
For reactions with allylic ethers or acetals: Didiuk MT, Morken JP, Hoveyda AH. J. Am. Chem. Soc. 1995;117:7273. Nomura N, RajanBabu TV. Tetrahedron Lett. 1997;38:1713. Didiuk MT, Morken JP, Hoveyda AH. Tetrahedron. 1998;54:1117. Weatherhead GS, Houser JH, Ford JG, Jamieson JY, Schrock RR, Hoveyda AH. Tetrahedron Lett. 2000;41:9553. For reactions with benzylic ethers, see: Taylor BLH, Swift EC, Waetzig JD, Jarvo ER. J. Am. Chem. Soc. 2011;133:389. Greene MA, Yonova IM, Williams FJ, Jarvo ER. Org. Lett. 2012;14:4293. Taylor BLH, Jarvo ER. Synlett. 2011:2761.
-
-
- Hartwig JF. Organotransition Metal Chemistry. University Science Books; Sausalito, CA: 2010.
-
- Louw WJ, de Waal DJA, Chapman JE. Chem. Commun. 1977:845.
- Crabtree RH, Quirk JM, Fillebeen-Khan T, Morris GE. J. Organomet. Chem. 1979;181:203.
-
Singleton and co-workers have reported a secondary carbon isotope effect for the oxidative addition of a tertiary allylic acetate to Pd that is consistent with an SN1-like mechanism: Singleton DA, Christian CF. Tetrahedron Lett. 2005;46:1631.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

