Preprotachykinin-A gene disruption attenuates nociceptive sensitivity after opioid administration and incision by peripheral and spinal mechanisms in mice
- PMID: 23031399
- PMCID: PMC3491068
- DOI: 10.1016/j.jpain.2012.07.009
Preprotachykinin-A gene disruption attenuates nociceptive sensitivity after opioid administration and incision by peripheral and spinal mechanisms in mice
Abstract
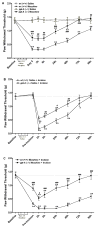
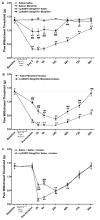
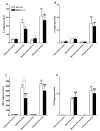
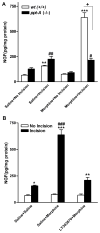
The preprotachykinin A gene (ppt-A) codes for Substance P (SP), supports nociceptive sensitization, and modulates inflammatory responses after incision. Repeated opioid use produces paradoxical pain sensitization-termed opioid-induced hyperalgesia (OIH) -which can exacerbate pain after incision. Here the contribution of SP to peri-incisional nociceptive sensitization and nociceptive mediator production after opioid treatment was examined utilizing ppt-A knockout (-/-) mice and the neurokinin (NK1) receptor antagonist LY303870. Less mechanical allodynia was observed in ppt-A(-/-) mice compared to wild types (wt) after morphine treatment both before and after incision. Moreover, LY303870 administered with morphine reduced incisional hyperalgesia in wt mice. Incision after saline or escalating morphine treatment upregulated skin IL-1β, IL-6, G-CSF and MIP-1α levels in ppt-A(-/-) and wt mice similarly. However, chronic morphine treatment greatly exacerbated increases in skin nerve growth factor levels after incision, an effect entirely dependent upon intact SP signaling. Additionally, SP dependent upregulation of prodynorphin, NMDA1 and NK1 receptor expression in spinal cord was seen after morphine treatment and incision. A similar pattern was seen for 5-HT3 receptor expression in tissue from dorsal root ganglia. Therefore, SP may work at both central and peripheral sites to enhance nociceptive sensitization after morphine treatment and incision.
Perspective: These studies show that SP signaling modulates enhanced nerve growth factor production and changes in neuronal gene expression seen after incision in mice previously exposed to morphine.
Copyright © 2012 American Pain Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Allen JW, Mantyh PW, Horais K, Tozier N, Rogers SD, Ghilardi JR, Cizkova D, Grafe MR, Richter P, Lappi DA, Yaksh TL. Safety evaluation of intrathecal substance P-saporin, a targeted neurotoxin, in dogs. Toxicol Sci. 2006;91:286–98. - PubMed
-
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–87. - PubMed
-
- Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. - PubMed
-
- Basbaum AI. Spinal mechanisms of acute and persistent pain. Reg Anesth Pain Med. 1999;24:59–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

