A randomized study to assess the immunogenicity, antibody persistence and safety of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in children aged 2-10 years
- PMID: 23032168
- PMCID: PMC3656081
- DOI: 10.4161/hv.22165
A randomized study to assess the immunogenicity, antibody persistence and safety of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in children aged 2-10 years
Abstract
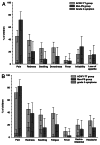
Incidence of meningococcal diseases is high in children, and effective vaccines are needed for this age group. In this phase II, open, controlled study, 309 children aged 2-10 y from Finland were randomized (3:1) into two parallel groups to receive one dose of meningococcal ACWY-tetanus toxoid conjugate vaccine (ACWY-TT group; n = 231) or a licensed meningococcal ACWY polysaccharide vaccine (Men-PS group; n = 78). Serum bactericidal activity using rabbit complement (rSBA) was evaluated up to three years post-vaccination. Exploratory comparisons suggested that rSBA vaccine response rates and geometric mean titers (GMTs) for each serogroup at one month post-vaccination and rSBA GMTs for serogroups A, W-135 and Y up to three years post-vaccination were higher in the ACWY-TT compared with Men-PS group, but did not detect any difference between groups in terms of rSBA-MenC GMTs at three years post-vaccination; this is explained by the higher proportion of children from the Men-PS group who were excluded because they were re-vaccinated with a monovalent meningococcal serogroup C vaccine due to loss of protective antibody levels against this serogroup. Although there was a higher incidence of local reactogenicity in the ACWY-TT group, general and unsolicited symptoms reporting rates were comparable in both groups. This study showed that MenACWY-TT was immunogenic with a clinically acceptable safety profile in children aged 2-10 y. MenACWY-TT induced higher functional antibody titers for all serogroups, which persisted longer for serogroups A, W-135 and Y, than the MenACWY polysaccharide vaccine.
Trial registration: ClinicalTrials.gov NCT00427908.
Keywords: bactericidal activity; child; conjugate vaccine; immunogenicity; persistence; polysaccharide vaccine; safety; tetravalent meningococcal vaccine.
Figures

References
-
- European Centre for Disease Prevention and Control. Annual epidemiological report. Reporting on 2009 surveillance data and 2010 epidemic intelligence data. Stockholm ECDC 2011; 155-57.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
