Subcellular characteristics of functional intracellular renin-angiotensin systems
- PMID: 23032352
- PMCID: PMC3770295
- DOI: 10.1016/j.peptides.2012.09.016
Subcellular characteristics of functional intracellular renin-angiotensin systems
Abstract
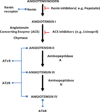
The renin-angiotensin system (RAS) is now regarded as an integral component in not only the development of hypertension, but also in physiologic and pathophysiologic mechanisms in multiple tissues and chronic disease states. While many of the endocrine (circulating), paracrine (cell-to-different cell) and autacrine (cell-to-same cell) effects of the RAS are believed to be mediated through the canonical extracellular RAS, a complete, independent and differentially regulated intracellular RAS (iRAS) has also been proposed. Angiotensinogen, the enzymes renin and angiotensin-converting enzyme (ACE) and the angiotensin peptides can all be synthesized and retained intracellularly. Angiotensin receptors (types I and 2) are also abundant intracellularly mainly at the nuclear and mitochondrial levels. The aim of this review is to focus on the most recent information concerning the subcellular localization, distribution and functions of the iRAS and to discuss the potential consequences of activation of the subcellular RAS on different organ systems.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Andrade MC, Quinto BM, Carmona AK, Ribas OS, Boim MA, Schor N, et al. Purification and characterization of angiotensin I-converting enzymes from mesangial cells in culture. J Hypertens. 1998;16:2063–2074. - PubMed
-
- Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. - PubMed
-
- Camargo de Andrade MC, DiMarco GS, de Paulo Castro Teixeira V, Mortara RA, Sabatini RA, Pasquero JB, et al. Expression and localization of N-domain ANG I-converting enzymes in mesangial cells in culture from spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F364–F375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

