Neurovascular coupling varies with level of global cerebral ischemia in a rat model
- PMID: 23032485
- PMCID: PMC3597370
- DOI: 10.1038/jcbfm.2012.137
Neurovascular coupling varies with level of global cerebral ischemia in a rat model
Abstract
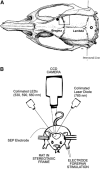
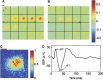
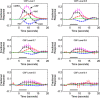
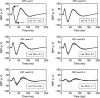
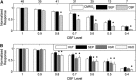
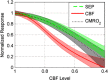
In this study, cerebral blood flow, oxygenation, metabolic, and electrical functional responses to forepaw stimulation were monitored in rats at different levels of global cerebral ischemia from mild to severe. Laser speckle contrast imaging and optical imaging of intrinsic signals were used to measure changes in blood flow and oxygenation, respectively, along with a compartmental model to calculate changes in oxygen metabolism from these measured changes. To characterize the electrical response to functional stimulation, we measured somatosensory evoked potentials (SEPs). Global graded ischemia was induced through unilateral carotid artery occlusion, bilateral carotid artery occlusion, bilateral carotid and right subclavian artery (SCA) occlusion, or carotid and SCA occlusion with negative lower body pressure. We found that the amplitude of the functional metabolic response remained tightly coupled to the amplitude of the SEP at all levels of ischemia observed. However, as the level of ischemia became more severe, the flow response was more strongly attenuated than the electrical response, suggesting that global ischemia was associated with an uncoupling between the functional flow and electrical responses.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

