Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients
- PMID: 23032743
- PMCID: PMC3525747
- DOI: 10.1158/1078-0432.CCR-12-1177
Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients
Abstract
Purpose: Adoptive cell therapy (ACT) using autologous tumor-infiltrating lymphocytes (TIL) is a promising treatment for metastatic melanoma unresponsive to conventional therapies. We report here on the results of an ongoing phase II clinical trial testing the efficacy of ACT using TIL in patients with metastatic melanoma and the association of specific patient clinical characteristics and the phenotypic attributes of the infused TIL with clinical response.
Experimental design: Altogether, 31 transiently lymphodepleted patients were treated with their expanded TIL, followed by two cycles of high-dose interleukin (IL)-2 therapy. The effects of patient clinical features and the phenotypes of the T cells infused on the clinical response were determined.
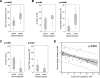
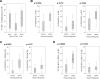
Results: Overall, 15 of 31 (48.4%) patients had an objective clinical response using immune-related response criteria (irRC) with 2 patients (6.5%) having a complete response. Progression-free survival of more than 12 months was observed for 9 of 15 (60%) of the responding patients. Factors significantly associated with the objective tumor regression included a higher number of TIL infused, a higher proportion of CD8(+) T cells in the infusion product, a more differentiated effector phenotype of the CD8(+) population, and a higher frequency of CD8(+) T cells coexpressing the negative costimulation molecule "B- and T-lymphocyte attenuator" (BTLA). No significant difference in the telomere lengths of TIL between responders and nonresponders was identified.
Conclusion: These results indicate that the immunotherapy with expanded autologous TIL is capable of achieving durable clinical responses in patients with metastatic melanoma and that CD8(+) T cells in the infused TIL, particularly differentiated effectors cells and cells expressing BTLA, are associated with tumor regression.
Figures

References
-
- Trinh VA. Current management of metastatic melanoma. Am J Health Syst Pharm. 2008;65:S3–8. - PubMed
-
- Quirt I, Verma S, Petrella T, Bak K, Charette M. Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist. 2007;12:1114–23. - PubMed
-
- Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol. 2008;20:183–9. - PubMed
-
- Flaherty KT, McArthur G. BRAF, a target in melanoma: implications for solid tumor drug development. Cancer. 2010;116:4902–13. - PubMed
-
- Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov. 10:811–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

