The possible "proton sponge " effect of polyethylenimine (PEI) does not include change in lysosomal pH
- PMID: 23032976
- PMCID: PMC3538306
- DOI: 10.1038/mt.2012.185
The possible "proton sponge " effect of polyethylenimine (PEI) does not include change in lysosomal pH
Abstract
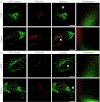
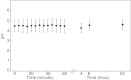
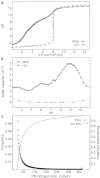
Polycations such as polyethylenimine (PEI) are used in many novel nonviral vector designs and there are continuous efforts to increase our mechanistic understanding of their interactions with cells. Even so, the mechanism of polyplex escape from the endosomal/lysosomal pathway after internalization is still elusive. The "proton sponge " hypothesis remains the most generally accepted mechanism, although it is heavily debated. This hypothesis is associated with the large buffering capacity of PEI and other polycations, which has been interpreted to cause an increase in lysosomal pH even though no conclusive proof has been provided. In the present study, we have used a nanoparticle pH sensor that was developed for pH measurements in the endosomal/lysosomal pathway. We have carried out quantitative measurements of lysosomal pH as a function of PEI content and correlate the results to the "proton sponge " hypothesis. Our measurements show that PEI does not induce change in lysosomal pH as previously suggested and quantification of PEI concentrations in lysosomes makes it uncertain that the "proton sponge " effect is the dominant mechanism of polyplex escape.
Figures



References
-
- Mintzer MA., and, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. - PubMed
-
- Brito L, Little S, Langer R., and, Amiji M. Poly(beta-amino ester) and cationic phospholipid-based lipopolyplexes for gene delivery and transfection in human aortic endothelial and smooth muscle cells. Biomacromolecules. 2008;9:1179–1187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

