Effect of adherence as measured by MEMS, ritonavir boosting, and CYP3A5 genotype on atazanavir pharmacokinetics in treatment-naive HIV-infected patients
- PMID: 23033116
- PMCID: PMC3939416
- DOI: 10.1038/clpt.2012.137
Effect of adherence as measured by MEMS, ritonavir boosting, and CYP3A5 genotype on atazanavir pharmacokinetics in treatment-naive HIV-infected patients
Abstract
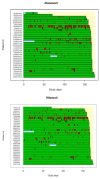
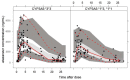
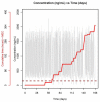
We investigated population pharmacokinetics and pharmacogenetics of ritonavir-boosted atazanavir (ATV), using drug intake times exactly recorded by the Medication Event Monitoring System. The ANRS 134-COPHAR 3 trial was conducted in 35 HIV-infected treatment-naive patients. ATV (300 mg), ritonavir (100 mg), and tenofovir (300 mg) + emtricitabine (200 mg), in bottles with MEMS caps, were taken once daily for 6 months. Six blood samples were collected at week 4 to measure drug concentrations, and trough levels were measured bimonthly. A model integrating ATV and ritonavir pharmacokinetics and pharmacogenetics used nonlinear mixed effects. Use of exact dosing data halved unexplained variability in ATV clearance. The ritonavir-ATV interaction model suggested that optimal boosting effect is achievable at lower ritonavir exposures. Patients with at least one copy of the CYP3A5*1 allele exhibited 28% higher oral clearance. We provide evidence that variability in ATV pharmacokinetics is defined by adherence, CYP3A5 genotype, and ritonavir exposure.
Conflict of interest statement
Figures



References
-
- Piliero PJ. Atazanavir: a novel HIV-1 protease inhibitor. Expert Opin Investig Drugs. 2002 Sep;11(9):1295–301. - PubMed
-
- BMS. Summary Product Characteristics. Bristol Myers Squibb; 2005. Reyataz (Atazanavir Sulfate) Capsules.
-
- Goldsmith DR, Perry CM. Atazanavir Drugs. 2003;63(16):1679–93. discussion 94–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

