Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing
- PMID: 23033146
- PMCID: PMC3502374
- DOI: 10.1093/infdis/jis607
Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing
Abstract
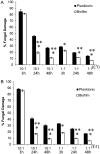
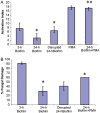
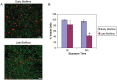
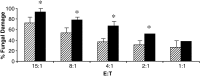
Neutrophils are found within Candida albicans biofilms in vivo and could play a crucial role in clearing the pathogen from biofilms forming on catheters and mucosal surfaces. Our goal was to compare the antimicrobial activity of neutrophils against developing and mature C. albicans biofilms and identify biofilm-specific properties mediating resistance to immune cells. Antibiofilm activity was measured with the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)2H-tetrazolium-5-carboxanilide assay and a molecular Candida viability assay. Reactive oxygen species generation was assessed by measuring fluorescence of 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester in preloaded neutrophils. We found that mature biofilms were resistant to leukocytic killing and did not trigger reactive oxygen species, even though neutrophils retained their viability and functional activation potential. Beta-glucans found in the extracellular matrix negatively affected antibiofilm activities. We conclude that these polymers act as a decoy mechanism to prevent neutrophil activation and that this represents an important innate immune evasion mechanism of C. albicans biofilms.
Figures
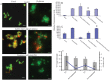
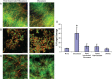
References
-
- Jesaitis AJ, Franklin MJ, Berglund D, et al. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol. 2003;171:4329–39. - PubMed
-
- Günther F, Wabnitz GH, Stroh P, et al. Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN) Mol Immunol. 2009;46:1805–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

