Identification and characterization of a compound that protects cardiac tissue from human Ether-à-go-go-related gene (hERG)-related drug-induced arrhythmias
- PMID: 23033485
- PMCID: PMC3501040
- DOI: 10.1074/jbc.M112.380162
Identification and characterization of a compound that protects cardiac tissue from human Ether-à-go-go-related gene (hERG)-related drug-induced arrhythmias
Abstract
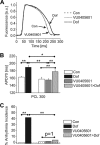
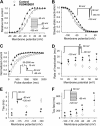
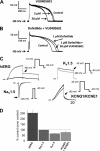
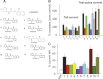
The human Ether-à-go-go-related gene (hERG)-encoded K(+) current, I(Kr) is essential for cardiac repolarization but is also a source of cardiotoxicity because unintended hERG inhibition by diverse pharmaceuticals can cause arrhythmias and sudden cardiac death. We hypothesized that a small molecule that diminishes I(Kr) block by a known hERG antagonist would constitute a first step toward preventing hERG-related arrhythmias and facilitating drug discovery. Using a high-throughput assay, we screened a library of compounds for agents that increase the IC(70) of dofetilide, a well characterized hERG blocker. One compound, VU0405601, with the desired activity was further characterized. In isolated, Langendorff-perfused rabbit hearts, optical mapping revealed that dofetilide-induced arrhythmias were reduced after pretreatment with VU0405601. Patch clamp analysis in stable hERG-HEK cells showed effects on current amplitude, inactivation, and deactivation. VU0405601 increased the IC(50) of dofetilide from 38.7 to 76.3 nM. VU0405601 mitigates the effects of hERG blockers from the extracellular aspect primarily by reducing inactivation, whereas most clinically relevant hERG inhibitors act at an inner pore site. Structure-activity relationships surrounding VU0405601 identified a 3-pyridiyl and a naphthyridine ring system as key structural components important for preventing hERG inhibition by multiple inhibitors. These findings indicate that small molecules can be designed to reduce the sensitivity of hERG to inhibitors.
Figures





References
-
- Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia. HERG encodes the IKr potassium channel. Cell 81, 299–307 - PubMed
-
- Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. (1995) HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269, 92–95 - PubMed
-
- Curran M. E., Splawski I., Timothy K. W., Vincent G. M., Green E. D., Keating M. T. (1995) A molecular basis for cardiac arrhythmia. HERG mutations cause long QT syndrome. Cell 80, 795–803 - PubMed
-
- Hedley P. L., Jørgensen P., Schlamowitz S., Wangari R., Moolman-Smook J., Brink P. A., Kanters J. K., Corfield V. A., Christiansen M. (2009) The genetic basis of long QT and short QT syndromes. A mutation update. Hum. Mutat. 30, 1486–1511 - PubMed
-
- Spector P. S., Curran M. E., Keating M. T., Sanguinetti M. C. (1996) Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ. Res. 78, 499–503 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

