Organization of the neural switching circuitry underlying reflex micturition
- PMID: 23033877
- PMCID: PMC3718009
- DOI: 10.1111/apha.12014
Organization of the neural switching circuitry underlying reflex micturition
Abstract
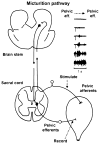
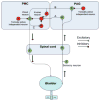
The functions of the lower urinary tract to store and periodically eliminate urine are regulated by a complex neural control system in the brain and spinal cord that coordinates the activity of the bladder and urethral outlet. Experimental studies in animals indicate that urine storage is modulated by reflex mechanisms in the spinal cord, whereas voiding is mediated by a spinobulbospinal pathway passing through a coordination centre in the rostral brain stem. Many of the neural circuits controlling micturition exhibit switch-like patterns of activity that turn on and off in an all-or-none manner. This study summarizes the anatomy and physiology of the spinal and supraspinal micturition switching circuitry and describes a computer model of these circuits that mimics the switching functions of the bladder and urethra at the onset of micturition.
© 2012 The Authors Acta Physiologica © 2012 Scandinavian Physiological Society.
Conflict of interest statement
The authors have no conflict of interest and nothing to disclose.
Figures



References
-
- Araki I, de Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J Neurophysiol. 1996;76:215–226. - PubMed
-
- Barrington F. The effect of lesions of the hind- and mid-brain on micturition in the cat. Quart J Exp Physiol. 1925;15:81–102.
-
- Bastiaanssen EH, van Leeuwen JL, Vanderschoot J, Redert PA. A myocybernetic model of the lower urinary tract. J Theor Biol. 1996;178:113–133. - PubMed
-
- Beckel JM, Holstege G. Neuroanatomy of the lower urinary tract. Handb Exp Pharmacol. 2011;202:99–116. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

