The microarray technology: facts and controversies
- PMID: 23034051
- PMCID: PMC7129321
- DOI: 10.1111/1469-0691.12024
The microarray technology: facts and controversies
Abstract
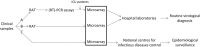
Molecular diagnostic techniques for viral testing have undergone rapid development in recent years. They are becoming more widely used than the classical virological assays in the majority of clinical virology laboratories, and now represent a new method for the diagnosis of human viral infections. Recently, new techniques based on multiplex RT-PCR amplification followed by microarray analysis have been developed and evaluated. On the basis of amplification of viral genome-specific fragments by multiplex RT-PCR and their subsequent detection via hybridization with microorganism-specific binding probes on solid surfaces, they allow simultaneous detection and identification of multiple viruses in a single clinical sample. The management of viral central nervous system and respiratory tract infections currently represents the two main applications of the microarrays in routine virological practice. Microarrays have shown reliable results in comparison with those of referenced (RT)-PCR assays, and appear to be of major interest for the detection of a broad range of respiratory and neurotropic viruses, assessment of the pathogenicity of newly discovered or neglected viruses, and identification of multiple viral infections in clinical samples. Despite several limitations observed during the different studies performed, this new technology might improve the clinical management of patients by enlarging the range of the viruses detected, in particular in cases of severe infections leading to patient hospitalization in the intensive-care unit. They might also help in the prevention of nosocomial transmission in hospital departments by contributing to the development of new epidemiological surveillance systems for viral infections.
© 2012 The Authors. Clinical Microbiology and Infection © 2012 European Society of Clinical Microbiology and Infectious Diseases.
Figures
Similar articles
-
Rapid detection of respiratory tract viral infections and coinfections in patients with influenza-like illnesses by use of reverse transcription-PCR DNA microarray systems.J Clin Microbiol. 2010 Nov;48(11):3836-42. doi: 10.1128/JCM.00733-10. Epub 2010 Aug 25. J Clin Microbiol. 2010. PMID: 20739481 Free PMC article.
-
Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system.J Med Virol. 2012 Jun;84(6):979-85. doi: 10.1002/jmv.23272. J Med Virol. 2012. PMID: 22499022 Free PMC article.
-
A novel multiplex PCR-electronic microarray assay for rapid and simultaneous detection of bovine respiratory and enteric pathogens.J Virol Methods. 2018 Nov;261:51-62. doi: 10.1016/j.jviromet.2018.08.010. Epub 2018 Aug 10. J Virol Methods. 2018. PMID: 30102924 Free PMC article.
-
Emerging molecular assays for detection and characterization of respiratory viruses.Clin Lab Med. 2009 Dec;29(4):673-93. doi: 10.1016/j.cll.2009.07.005. Clin Lab Med. 2009. PMID: 19892228 Free PMC article. Review.
-
[Development of multi-pathogen detection techniques for respiratory viruses].Bing Du Xue Bao. 2013 Mar;29(2):238-44. Bing Du Xue Bao. 2013. PMID: 23757859 Review. Chinese.
Cited by
-
Cancer microarray data feature selection using multi-objective binary particle swarm optimization algorithm.EXCLI J. 2016 Aug 1;15:460-473. doi: 10.17179/excli2016-481. eCollection 2016. EXCLI J. 2016. PMID: 27822174 Free PMC article.
-
Viruses Infecting Trees and Herbs That Produce Edible Fleshy Fruits with a Prominent Value in the Global Market: An Evolutionary Perspective.Plants (Basel). 2022 Jan 13;11(2):203. doi: 10.3390/plants11020203. Plants (Basel). 2022. PMID: 35050091 Free PMC article. Review.
-
Mixed viral infections circulating in hospitalized patients with respiratory tract infections in kuwait.Adv Virol. 2015;2015:714062. doi: 10.1155/2015/714062. Epub 2015 Apr 23. Adv Virol. 2015. PMID: 25983755 Free PMC article.
-
New Approaches to Comparative and Animal Stress Biology Research in the Post-genomic Era: A Contextual Overview.Comput Struct Biotechnol J. 2014 Sep 30;11(19):138-46. doi: 10.1016/j.csbj.2014.09.006. eCollection 2014 Sep. Comput Struct Biotechnol J. 2014. PMID: 25408848 Free PMC article. Review.
-
FilmArray® Meningoencephalitis panel in the diagnosis of central nervous system infections: stewardship and cost analysis in a paediatric hospital in Chile.BMC Pediatr. 2022 Apr 5;22(1):182. doi: 10.1186/s12887-022-03241-1. BMC Pediatr. 2022. PMID: 35382778 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources