Helminth infections and host immune regulation
- PMID: 23034321
- PMCID: PMC3485755
- DOI: 10.1128/CMR.05040-11
Helminth infections and host immune regulation
Abstract
Helminth parasites infect almost one-third of the world's population, primarily in tropical regions. However, regions where helminth parasites are endemic record much lower prevalences of allergies and autoimmune diseases, suggesting that parasites may protect against immunopathological syndromes. Most helminth diseases are spectral in nature, with a large proportion of relatively asymptomatic cases and a subset of patients who develop severe pathologies. The maintenance of the asymptomatic state is now recognized as reflecting an immunoregulatory environment, which may be promoted by parasites, and involves multiple levels of host regulatory cells and cytokines; a breakdown of this regulation is observed in pathological disease. Currently, there is much interest in whether helminth-associated immune regulation may ameliorate allergy and autoimmunity, with investigations in both laboratory models and human trials. Understanding and exploiting the interactions between these parasites and the host regulatory network are therefore likely to highlight new strategies to control both infectious and immunological diseases.
Figures

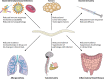
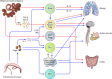


References
-
- Allen JE, Lawrence RA, Maizels RM. 1996. Antigen presenting cells from mice harboring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int. Immunol. 8:143–151 - PubMed
-
- Allen JE, Maizels RM. 2011. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11:375–388 - PubMed
-
- Amu S, et al. 2010. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 125:1114–1124 - PubMed
-
- Anderson RM, May RM. 1985. Helminth infection of humans: mathematical model, population dynamics and control. Adv. Parasitol. 24:1–101 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

