K+-Cl- cotransporter-2 KCC2 in chicken cardiomyocytes
- PMID: 23034386
- PMCID: PMC3530769
- DOI: 10.1152/ajpcell.00274.2012
K+-Cl- cotransporter-2 KCC2 in chicken cardiomyocytes
Abstract
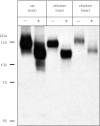
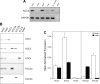
Using antibodies prepared against a unique region (exon 22-24) of rat K(+)-Cl(-) cotransporter-2 (KCC2), we confirmed that the ~140-kDa KCC2 protein is exclusively expressed in rat brain, but in chicken, we observed strong reactivity not only with the ~140-kDa KCC2 protein in brain but also a slightly larger ~145-kDa protein in heart. In silico analysis showed that while exon 22 of KCC2 is unique to this isoform in therian mammals, it is retained in KCC2's closest paralog, KCC4, of lower vertebrates, including chicken. To eliminate potential cross-reactivity with chicken KCC4, the antibodies were preadsorbed with blocking peptides prepared over the only two regions showing significant sequence identity to chicken KCC4. This completely eliminated antibody recognition of exogenously expressed chicken KCC4 but not of the ~145-kDa protein in chicken heart, indicating that chicken heart expresses KCC2. Real-time PCR confirmed robust KCC2 transcript expression in both chicken brain and heart. Chicken heart expressed predominantly the longer KCC2a splice variant consistent with the larger ~145-kDa protein in chicken heart. Immunofluorescence microscopy revealed prominent plasma membrane KCC2 labeling in chicken ventricular cardiomyocytes. We hypothesize that KCC2 is an important Cl(-) extrusion pathway in avian cardiomyocytes that counters channel-mediated Cl(-) loading during high heart rates with β-adrenergic stimulation. While KCC2 is absent from mammalian cardiomyocytes, understanding the role that the other KCC isoforms play in Cl(-) homeostasis of these cells represents a nascent area of research.
Figures







References
-
- Baumgarten CM, Fozzard HA. Intracellular chloride activity in mammalian ventricular muscle. Am J Physiol Cell Physiol 241: C121–C129, 1981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

