Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension
- PMID: 23034390
- PMCID: PMC3532492
- DOI: 10.1152/ajpcell.00044.2012
Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension
Abstract
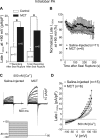
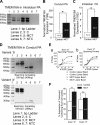
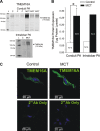
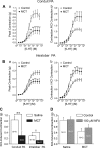
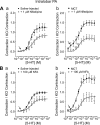
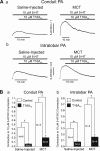
Pulmonary artery smooth muscle cells (PASMCs) are more depolarized and display higher Ca(2+) levels in pulmonary hypertension (PH). Whether the functional properties and expression of Ca(2+)-activated Cl- channels (Cl(Ca)), an important excitatory mechanism in PASMCs, are altered in PH is unknown. The potential role of Cl(Ca) channels in PH was investigated using the monocrotaline (MCT)-induced PH model in the rat. Three weeks postinjection with a single dose of MCT (50 mg/kg ip), the animals developed right ventricular hypertrophy (heart weight measurements) and changes in pulmonary arterial flow (pulse-waved Doppler imaging) that were consistent with increased pulmonary arterial pressure and PH. Whole cell patch experiments revealed an increase in niflumic acid (NFA)-sensitive Ca(2+)-activated Cl(-) current [I(Cl(Ca))] density in PASMCs from large conduit and small intralobar pulmonary arteries of MCT-treated rats vs. aged-matched saline-injected controls. Quantitative RT-PCR and Western blot analysis revealed that the alterations in I(Cl(Ca)) were accompanied by parallel changes in the expression of TMEM16A, a gene recently shown to encode for Cl(Ca) channels. The contraction to serotonin of conduit and intralobar pulmonary arteries from MCT-treated rats exhibited greater sensitivity to nifedipine (1 μM), an l-type Ca(2+) channel blocker, and NFA (30 or 100 μM, with or without 10 μM indomethacin to inhibit cyclooxygenases) or T16A(Inh)-A01 (10 μM), TMEM16A/Cl(Ca) channel inhibitors, than that of control animals. In conclusion, augmented Cl(Ca)/TMEM16A channel activity is a major contributor to the changes in electromechanical coupling of PA in this model of PH. TMEM16A-encoded channels may therefore represent a novel therapeutic target in this disease.
Figures



References
-
- Altiere RJ, Olson JW, Gillespie MN. Altered pulmonary vascular smooth muscle responsiveness in monocrotaline-induced pulmonary hypertension. J Pharmacol Exp Ther 236: 390–395, 1986 - PubMed
-
- Angermann JE, Forrest AS, Greenwood IA, Leblanc N. Activation of Ca2+-activated Cl− channels by store-operated Ca2+ entry in arterial smooth muscle cells does not require reverse-mode Na+/Ca2+ exchange. Can J Physiol Pharmacol 90: 903–921, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

