APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19
- PMID: 23034631
- PMCID: PMC3478687
- DOI: 10.1242/dev.085407
APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19
Abstract
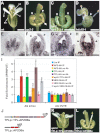
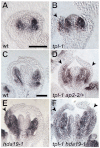
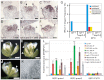
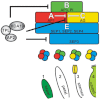
The development and coordination of complex tissues in eukaryotes requires precise spatial control of fate-specifying genes. Although investigations of such control have traditionally focused on mechanisms of transcriptional activation, transcriptional repression has emerged as being equally important in the establishment of gene expression territories. In the angiosperm flower, specification of lateral organ fate relies on the spatial regulation of the ABC floral organ identity genes. Our understanding of how the boundaries of these expression domains are controlled is not complete. Here, we report that the A-class organ identity gene APETALA2 (AP2), which is known to repress the C-class gene AGAMOUS, also regulates the expression borders of the B-class genes APETALA3 and PISTILLATA, and the E-class gene SEPALLATA3. We show that AP2 represses its target genes by physically recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. These results demonstrate that AP2 plays a broad role in flower development by controlling the expression domains of numerous floral organ identity genes.
Figures



References
-
- Alonso J. M., Stepanova A. N., Leisse T. J., Kim C. J., Chen H., Shinn P., Stevenson D. K., Zimmerman J., Barajas P., Cheuk R., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653-657 - PubMed
-
- Bowler C., Benvenuto G., Laflamme P., Molino D., Probst A. V., Tariq M., Paszkowski J. (2004). Chromatin techniques for plant cells. Plant J. 39, 776-789 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

