Dopaminergic neurons inhibit striatal output through non-canonical release of GABA
- PMID: 23034651
- PMCID: PMC3944587
- DOI: 10.1038/nature11466
Dopaminergic neurons inhibit striatal output through non-canonical release of GABA
Abstract
The substantia nigra pars compacta and ventral tegmental area contain the two largest populations of dopamine-releasing neurons in the mammalian brain. These neurons extend elaborate projections in the striatum, a large subcortical structure implicated in motor planning and reward-based learning. Phasic activation of dopaminergic neurons in response to salient or reward-predicting stimuli is thought to modulate striatal output through the release of dopamine to promote and reinforce motor action. Here we show that activation of dopamine neurons in striatal slices rapidly inhibits action potential firing in both direct- and indirect-pathway striatal projection neurons through vesicular release of the inhibitory transmitter GABA (γ-aminobutyric acid). GABA is released directly from dopaminergic axons but in a manner that is independent of the vesicular GABA transporter VGAT. Instead, GABA release requires activity of the vesicular monoamine transporter VMAT2, which is the vesicular transporter for dopamine. Furthermore, VMAT2 expression in GABAergic neurons lacking VGAT is sufficient to sustain GABA release. Thus, these findings expand the repertoire of synaptic mechanisms used by dopamine neurons to influence basal ganglia circuits, show a new substrate whose transport is dependent on VMAT2 and demonstrate that GABA can function as a bona fide co-transmitter in monoaminergic neurons.
Figures
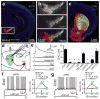
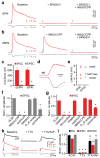
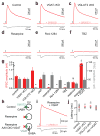

Comment in
-
Neuroscience: Promiscuous vesicles.Nature. 2012 Oct 11;490(7419):178-9. doi: 10.1038/490178a. Nature. 2012. PMID: 23060181 No abstract available.
-
Neurotransmission: GABA calls stop in the striatum.Nat Rev Neurosci. 2012 Dec;13(12):815. doi: 10.1038/nrn3382. Epub 2012 Oct 25. Nat Rev Neurosci. 2012. PMID: 23095838 No abstract available.
References
-
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80 (1):1–27. - PubMed
-
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13 (6):685–690. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12 (10):366–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

