Schwann cell glycogen selectively supports myelinated axon function
- PMID: 23034913
- PMCID: PMC3464962
- DOI: 10.1002/ana.23607
Schwann cell glycogen selectively supports myelinated axon function
Abstract
Objective: Interruption of energy supply to peripheral axons is a cause of axon loss. We determined whether glycogen was present in mammalian peripheral nerve, and whether it supported axon conduction during aglycemia.
Methods: We used biochemical assay and electron microscopy to determine the presence of glycogen, and electrophysiology to monitor axon function.
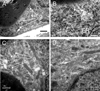
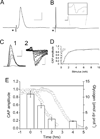
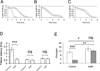
Results: Glycogen was present in sciatic nerve, its concentration varying directly with ambient glucose. Electron microscopy detected glycogen granules primarily in myelinating Schwann cell cytoplasm, and these diminished after exposure to aglycemia. During aglycemia, conduction failure in large myelinated axons (A fibers) mirrored the time course of glycogen loss. Latency to compound action potential (CAP) failure was directly related to nerve glycogen content at aglycemia onset. Glycogen did not benefit the function of slow-conducting, small-diameter unmyelinated axons (C fibers) during aglycemia. Blocking glycogen breakdown pharmacologically accelerated CAP failure during aglycemia in A fibers, but not in C fibers. Lactate was as effective as glucose in supporting sciatic nerve function, and was continuously released into the extracellular space in the presence of glucose and fell rapidly during aglycemia.
Interpretation: Our findings indicated that glycogen is present in peripheral nerve, primarily in myelinating Schwann cells, and exclusively supports large-diameter, myelinated axon conduction during aglycemia. Available evidence suggests that peripheral nerve glycogen breaks down during aglycemia and is passed, probably as lactate, to myelinated axons to support function. Unmyelinated axons are not protected by glycogen and are more vulnerable to dysfunction during periods of hypoglycemia. .
Copyright © 2012 American Neurological Association.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

