Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices
- PMID: 23035077
- PMCID: PMC3485081
- DOI: 10.1523/JNEUROSCI.2581-12.2012
Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices
Abstract
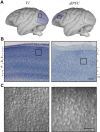
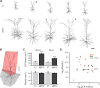
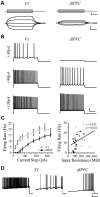
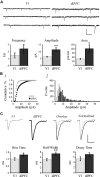
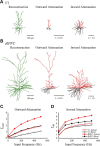
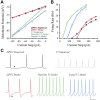
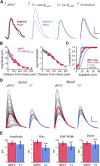
Whole-cell patch-clamp recordings and high-resolution 3D morphometric analyses of layer 3 pyramidal neurons in in vitro slices of monkey primary visual cortex (V1) and dorsolateral granular prefrontal cortex (dlPFC) revealed that neurons in these two brain areas possess highly distinctive structural and functional properties. Area V1 pyramidal neurons are much smaller than dlPFC neurons, with significantly less extensive dendritic arbors and far fewer dendritic spines. Relative to dlPFC neurons, V1 neurons have a significantly higher input resistance, depolarized resting membrane potential, and higher action potential (AP) firing rates. Most V1 neurons exhibit both phasic and regular-spiking tonic AP firing patterns, while dlPFC neurons exhibit only tonic firing. Spontaneous postsynaptic currents are lower in amplitude and have faster kinetics in V1 than in dlPFC neurons, but are no different in frequency. Three-dimensional reconstructions of V1 and dlPFC neurons were incorporated into computational models containing Hodgkin-Huxley and AMPA receptor and GABA(A) receptor gated channels. Morphology alone largely accounted for observed passive physiological properties, but led to AP firing rates that differed more than observed empirically, and to synaptic responses that opposed empirical results. Accordingly, modeling predicts that active channel conductances differ between V1 and dlPFC neurons. The unique features of V1 and dlPFC neurons are likely fundamental determinants of area-specific network behavior. The compact electrotonic arbor and increased excitability of V1 neurons support the rapid signal integration required for early processing of visual information. The greater connectivity and dendritic complexity of dlPFC neurons likely support higher level cognitive functions including working memory and planning.
Figures





References
-
- Acker CD, White JA. Roles of IA and morphology in action potential propagation in CA1 pyramidal cell dendrites. J Comput Neurosci. 2007;23:201–216. - PubMed
-
- Ascoli GA. Passive dendritic integration heavily affects spiking dynamics of recurrent networks. Neural Netw. 2003;16:657–663. - PubMed
-
- Barbas H. Architecture and cortical connections of the prefrontal cortex in the rhesus monkey. Adv Neurol. 1992;57:91–115. - PubMed
-
- Brown TH, Zador A, Mainen ZF, Claiborne BJ. Hebbian computations in hippocampal dendrites and spines. In: McKenna T, Davis J, Zornetzer SF, editors. Single neuron computation. San Diego: Academic; 1992. pp. 81–116.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
