Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer's disease model mice
- PMID: 23035082
- PMCID: PMC3475870
- DOI: 10.1523/JNEUROSCI.2107-12.2012
Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide rescues synaptic plasticity and memory deficits in Alzheimer's disease model mice
Abstract
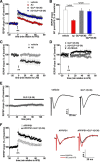
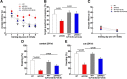
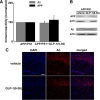
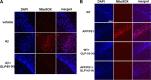
Glucagon-like peptide-1 (GLP-1) is an endogenous intestinal peptide that enhances glucose-stimulated insulin secretion. Its natural cleavage product GLP-1(9-36)(amide) possesses distinct properties and does not affect insulin secretion. Here we report that pretreatment of hippocampal slices with GLP-1(9-36)(amide) prevented impaired long-term potentiation (LTP) and enhanced long-term depression induced by exogenous amyloid β peptide Aβ((1-42)). Similarly, hippocampal LTP impairments in amyloid precursor protein/presenilin 1 (APP/PS1) mutant mice that model Alzheimer's disease (AD) were prevented by GLP-1(9-36)(amide). In addition, treatment of APP/PS1 mice with GLP-1(9-36)(amide) at an age at which they display impaired spatial and contextual fear memory resulted in a reversal of their memory defects. At the molecular level, GLP-1(9-36)(amide) reduced elevated levels of mitochondrial-derived reactive oxygen species and restored dysregulated Akt-glycogen synthase kinase-3β signaling in the hippocampus of APP/PS1 mice. Our findings suggest that GLP-1(9-36)(amide) treatment may have therapeutic potential for AD and other diseases associated with cognitive dysfunction.
Figures

References
-
- Alvarez E, Martínez MD, Roncero I, Chowen JA, García-Cuartero B, Gispert JD, Sanz C, Vázquez P, Maldonado A, de Cáceres J, Desco M, Pozo MA, Blázquez E. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798–806. - PubMed
-
- Alzheimer's Association. Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–244. - PubMed
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. - PubMed
-
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
-
- Ban K, Kim KH, Cho CK, Sauvé M, Diamandis EP, Backx PH, Drucker DJ, Husain M. Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9–39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151:1520–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
