Microtubule plus-end tracking protein CLASP2 regulates neuronal polarity and synaptic function
- PMID: 23035100
- PMCID: PMC3489028
- DOI: 10.1523/JNEUROSCI.2108-12.2012
Microtubule plus-end tracking protein CLASP2 regulates neuronal polarity and synaptic function
Abstract
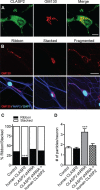
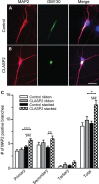
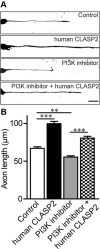
Microtubule organization and dynamics are essential during axon and dendrite formation and maintenance in neurons. However, little is known about the regulation of microtubule dynamics during synaptic development and function in mammalian neurons. Here, we present evidence that the microtubule plus-end tracking protein CLASP2 (cytoplasmic linker associated protein 2) is a key regulator of axon and dendrite outgrowth that leads to functional alterations in synaptic activity and formation. We found that CLASP2 protein levels steadily increase throughout neuronal development in the mouse brain and are specifically enriched at the growth cones of extending neurites. The short-hairpin RNA-mediated knockdown of CLASP2 in primary mouse neurons decreased axon and dendritic length, whereas overexpression of human CLASP2 caused the formation of multiple axons, enhanced dendritic branching, and Golgi condensation, implicating CLASP2 in neuronal morphogenesis. In addition, the CLASP2-induced morphological changes led to significant functional alterations in synaptic transmission. CLASP2 overexpression produced a large increase in spontaneous miniature event frequency that was specific to excitatory neurotransmitter release. The changes in presynaptic activity produced by CLASP2 overexpression were accompanied by increases in presynaptic terminal circumference, total synapse number, and a selective increase in presynaptic proteins that are involved in neurotransmitter release. Also, we found a smaller increase in miniature event amplitude that was accompanied by an increase in postsynaptic surface expression of GluA1 receptor localization. Together, these results provide evidence for involvement of the microtubule plus-end tracking protein CLASP2 in cytoskeleton-related mechanisms underlying neuronal polarity and interplay between microtubule stabilization and synapse formation and activity.
Figures





References
-
- Akhmanova A, Hoogenraad CC. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol. 2005;17:47–54. - PubMed
-
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. - PubMed
-
- Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, Galjart N. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–935. - PubMed
-
- Akhmanova A, Mausset-Bonnefont AL, van Cappellen W, Keijzer N, Hoogenraad CC, Stepanova T, Drabek K, van der Wees J, Mommaas M, Onderwater J, van der Meulen H, Tanenbaum ME, Medema RH, Hoogerbrugge J, Vreeburg J, Uringa EJ, Grootegoed JA, Grosveld F, Galjart N. The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev. 2005;19:2501–2515. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
