Hypoxia/reoxygenation impairs memory formation via adenosine-dependent activation of caspase 1
- PMID: 23035103
- PMCID: PMC3476834
- DOI: 10.1523/JNEUROSCI.0704-12.2012
Hypoxia/reoxygenation impairs memory formation via adenosine-dependent activation of caspase 1
Abstract
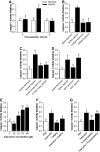
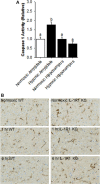
After hypoxia, a critical adverse outcome is the inability to create new memories. How anterograde amnesia develops or resolves remains elusive, but a link to brain-based IL-1 is suggested due to the vital role of IL-1 in both learning and brain injury. We examined memory formation in mice exposed to acute hypoxia. After reoxygenation, memory recall recovered faster than memory formation, impacting novel object recognition and cued fear conditioning but not spatially cued Y-maze performance. The ability of mice to form new memories after hypoxia/reoxygenation was accelerated in IL-1 receptor 1 knockout (IL-1R1 KO) mice, in mice receiving IL-1 receptor antagonist (IL-1RA), and in mice given the caspase 1 inhibitor Ac-YVAD-CMK. Mechanistically, hypoxia/reoxygenation more than doubled caspase 1 activity in the brain, which was localized to the amygdala compared to the hippocampus. This reoxygenation-dependent activation of caspase 1 was prevented by broad-spectrum adenosine receptor (AR) antagonism with caffeine and by targeted A1/A2A AR antagonism with 8-cyclopentyl-1,3-dipropylxanthine plus 3,7-dimethyl-1-propargylxanthine. Additionally, perfusion of adenosine activated caspase 1 in the brain, while caffeine blocked this action by adenosine. Finally, resolution of anterograde amnesia was improved by both caffeine and by targeted A1/A2A AR antagonism. These findings indicate that amygdala-based anterograde amnesia after hypoxia/reoxygenation is sustained by IL-1β generated through adenosine-dependent activation of caspase 1 after reoxygenation.
Figures




Similar articles
-
Adenosine through the A2A adenosine receptor increases IL-1β in the brain contributing to anxiety.Brain Behav Immun. 2014 Oct;41:218-31. doi: 10.1016/j.bbi.2014.05.018. Epub 2014 Jun 4. Brain Behav Immun. 2014. PMID: 24907587 Free PMC article.
-
Handling stress impairs learning through a mechanism involving caspase-1 activation and adenosine signaling.Brain Behav Immun. 2019 Aug;80:763-776. doi: 10.1016/j.bbi.2019.05.025. Epub 2019 May 17. Brain Behav Immun. 2019. PMID: 31108171 Free PMC article.
-
Effect of Ado A1- and A2-receptor activation on ventricular fibrillation during hypoxia-reoxygenation.Am J Physiol. 1994 Oct;267(4 Pt 2):H1447-54. doi: 10.1152/ajpheart.1994.267.4.H1447. Am J Physiol. 1994. PMID: 7943390
-
IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice.J Clin Invest. 2012 Oct;122(10):3476-89. doi: 10.1172/JCI60777. Epub 2012 Sep 4. J Clin Invest. 2012. PMID: 22945633 Free PMC article. Review.
-
Caffeine and adenosine.J Alzheimers Dis. 2010;20 Suppl 1:S3-15. doi: 10.3233/JAD-2010-1379. J Alzheimers Dis. 2010. PMID: 20164566 Review.
Cited by
-
Short-Term High-Fat Diet (HFD) Induced Anxiety-Like Behaviors and Cognitive Impairment Are Improved with Treatment by Glyburide.Front Behav Neurosci. 2016 Aug 11;10:156. doi: 10.3389/fnbeh.2016.00156. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27563288 Free PMC article.
-
The Effects of Hypoxia and Inflammation on Synaptic Signaling in the CNS.Brain Sci. 2016 Feb 17;6(1):6. doi: 10.3390/brainsci6010006. Brain Sci. 2016. PMID: 26901230 Free PMC article. Review.
-
Mechanisms of chemotherapy-induced behavioral toxicities.Front Neurosci. 2015 Apr 21;9:131. doi: 10.3389/fnins.2015.00131. eCollection 2015. Front Neurosci. 2015. PMID: 25954147 Free PMC article. Review.
-
Pifithrin-μ Prevents Cisplatin-Induced Chemobrain by Preserving Neuronal Mitochondrial Function.Cancer Res. 2017 Feb 1;77(3):742-752. doi: 10.1158/0008-5472.CAN-16-1817. Epub 2016 Nov 22. Cancer Res. 2017. PMID: 27879267 Free PMC article.
-
Modulation of neuroimmunity by adenosine and its receptors: metabolism to mental illness.Metabolism. 2014 Dec;63(12):1491-8. doi: 10.1016/j.metabol.2014.09.003. Epub 2014 Sep 26. Metabolism. 2014. PMID: 25308443 Free PMC article. Review.
References
-
- Avivi A, Brodsky L, Nevo E, Band MR. Differential expression profiling of the blind subterranean mole rat Spalax ehrenbergi superspecies: bioprospecting for hypoxia tolerance. Physiol Genomics. 2006;27:54–64. - PubMed
-
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. - PMC - PubMed
-
- Beatty WW, Salmon DP, Bernstein N, Martone M, Lyon L, Butters N. Procedural learning in a patient with amnesia due to hypoxia. Brain Cogn. 1987;6:386–402. - PubMed
-
- Berggren D, Gustafson Y, Eriksson B, Bucht G, Hansson LI, Reiz S, Winblad B. Postoperative confusion after anesthesia in elderly patients with femoral neck fractures. Anesth Analg. 1987;66:497–504. - PubMed
-
- Bickler PE, Hansen BM. Causes of calcium accumulation in rat cortical brain slices during hypoxia and ischemia: role of ion channels and membrane damage. Brain Res. 1994;665:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
