Population response to natural images in the primary visual cortex encodes local stimulus attributes and perceptual processing
- PMID: 23035105
- PMCID: PMC6704786
- DOI: 10.1523/JNEUROSCI.1596-12.2012
Population response to natural images in the primary visual cortex encodes local stimulus attributes and perceptual processing
Abstract
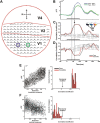
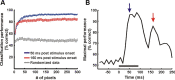
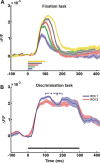
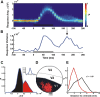
The primary visual cortex (V1) is extensively studied with a large repertoire of stimuli, yet little is known about its encoding of natural images. Using voltage-sensitive dye imaging in behaving monkeys, we measured neural population response evoked in V1 by natural images presented during a face/scramble discrimination task. The population response showed two distinct phases of activity: an early phase that was spread over most of the imaged area, and a late phase that was spatially confined. To study the detailed relation between the stimulus and the population response, we used a simple encoding model to compute a continuous map of the expected neural response based on local attributes of the stimulus (luminance and contrast), followed by an analytical retinotopic transformation. Then, we computed the spatial correlation between the maps of the expected and observed response. We found that the early response was highly correlated with the local luminance of the stimulus and was sufficient to effectively discriminate between stimuli at the single trial level. The late response, on the other hand, showed a much lower correlation to the local luminance, was confined to central parts of the face images, and was highly correlated with the animal's perceptual report. Our study reveals a continuous spatial encoding of low- and high-level features of natural images in V1. The low level is directly linked to the stimulus basic local attributes and the high level is correlated with the perceptual outcome of the stimulus processing.
Figures




References
-
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. - PubMed
-
- Albrecht DG, Geisler WS, Crane AM. Nonlinear properties of visual cortex neurons: temporal dynamics, stimulus, selectivity, neural performance. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge: MIT; 2004. pp. 747–764.
-
- Arieli A, Grinvald A, Slovin H. Dural substitute for long-term imaging of cortical activity in behaving monkeys and its clinical implications. J Neurosci Methods. 2002;114:119–133. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
