The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease
- PMID: 23035175
- PMCID: PMC3535858
- DOI: 10.1128/CVI.00364-12
The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease
Abstract
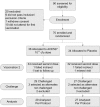
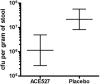
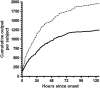
An oral, live attenuated, three-strain recombinant bacterial vaccine, ACE527, was demonstrated to generate strong immune responses to colonization factor and toxin antigens of enterotoxigenic Escherichia coli (ETEC) in human volunteers. The vaccine was safe and well tolerated at doses of up to 10(11) CFU, administered in each of two doses given 21 days apart. These observations have now been extended in a phase 2b study with a total of 70 subjects. Fifty-six of these subjects were challenged 28 days after the second dose of vaccine with the highly virulent ETEC strain H10407 to obtain preliminary indicators of efficacy against disease and to support further development of the vaccine for both travelers and infants in countries where ETEC is endemic. The vaccine had a significant impact on intestinal colonization by the challenge strain, as measured by quantitative fecal culture 2 days after challenge, demonstrating the induction of a functional immune response to the CFA/I antigen. The incidence and severity of diarrhea were also reduced in vaccinees as measured by a number of secondary and ad hoc endpoints, although the 27% reduction seen in the primary endpoint, moderate to severe diarrhea, was not statistically significant. Together, these observations support the hypothesis that the ACE527 vaccine has a dual mode of action, targeting both colonization factors and the heat-labile enterotoxin (LT), and suggest that it should be further developed for more advanced trials to evaluate its impact on the burden of ETEC disease in field settings.
Figures



Similar articles
-
Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge.Vaccine. 2019 Mar 28;37(14):1978-1986. doi: 10.1016/j.vaccine.2019.02.025. Epub 2019 Feb 21. Vaccine. 2019. PMID: 30797634 Free PMC article.
-
Immunogenicity and protective efficacy of a single-dose live non-pathogenic Escherichia coli oral vaccine against F4-positive enterotoxigenic Escherichia coli challenge in pigs.Vaccine. 2017 Jan 5;35(2):353-360. doi: 10.1016/j.vaccine.2016.11.045. Epub 2016 Dec 1. Vaccine. 2017. PMID: 27916413 Clinical Trial.
-
Attenuated Escherichia coli strains expressing the colonization factor antigen I (CFA/I) and a detoxified heat-labile enterotoxin (LThK63) enhance clearance of ETEC from the lungs of mice and protect mice from intestinal ETEC colonization and LT-induced fluid accumulation.Vet Immunol Immunopathol. 2013 Mar 15;152(1-2):57-67. doi: 10.1016/j.vetimm.2012.10.001. Epub 2012 Oct 5. Vet Immunol Immunopathol. 2013. PMID: 23122616
-
Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans.Expert Rev Vaccines. 2012 Jun;11(6):677-94. doi: 10.1586/erv.12.37. Expert Rev Vaccines. 2012. PMID: 22873126 Review.
-
Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it.Microb Pathog. 2018 Apr;117:162-169. doi: 10.1016/j.micpath.2018.02.032. Epub 2018 Feb 21. Microb Pathog. 2018. PMID: 29474827 Review.
Cited by
-
Protective Enterotoxigenic Escherichia coli Antigens in a Murine Intranasal Challenge Model.PLoS Negl Trop Dis. 2015 Aug 5;9(8):e0003924. doi: 10.1371/journal.pntd.0003924. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26244636 Free PMC article.
-
Development of an enterotoxigenic Escherichia coli vaccine based on the heat-stable toxin.Hum Vaccin Immunother. 2019;15(6):1379-1388. doi: 10.1080/21645515.2018.1496768. Epub 2018 Aug 21. Hum Vaccin Immunother. 2019. PMID: 30081709 Free PMC article. Review.
-
Development of a novel multiplex electrochemiluminescent-based immunoassay to aid enterotoxigenic Escherichia coli vaccine development and evaluations.J Immunol Methods. 2019 Jul;470:6-14. doi: 10.1016/j.jim.2019.04.003. Epub 2019 Apr 18. J Immunol Methods. 2019. PMID: 31004579 Free PMC article.
-
Refinement of the CS6-expressing enterotoxigenic Escherichia coli strain B7A human challenge model: A randomized trial.PLoS One. 2020 Dec 2;15(12):e0239888. doi: 10.1371/journal.pone.0239888. eCollection 2020. PLoS One. 2020. PMID: 33264302 Free PMC article. Clinical Trial.
-
A combined vaccine approach against Vibrio cholerae and ETEC based on outer membrane vesicles.Front Microbiol. 2015 Aug 11;6:823. doi: 10.3389/fmicb.2015.00823. eCollection 2015. Front Microbiol. 2015. PMID: 26322032 Free PMC article.
References
-
- Black RE, et al. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987 - PubMed
-
- Carpenter CM, et al. 2006. Comparison of the antibody in lymphocyte supernatant (ALS) and ELISPOT assays for detection of mucosal immune responses to antigens of enterotoxigenic Escherichia coli in challenged and vaccinated volunteers. Vaccine 24:3709–3718 - PubMed
-
- Collett D. 2002. Modelling binary data, 2nd ed Chapman & Hall/CRC, Boca Raton, FL
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

