Recognition of the different structural forms of the capsid protein determines the outcome following infection with porcine circovirus type 2
- PMID: 23035215
- PMCID: PMC3503079
- DOI: 10.1128/JVI.01763-12
Recognition of the different structural forms of the capsid protein determines the outcome following infection with porcine circovirus type 2
Abstract
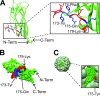
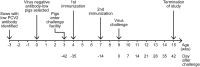
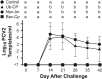
Porcine circovirus type 2 (PCV2) capsid protein (CP) is the only protein necessary for the formation of the virion capsid, and recombinant CP spontaneously forms virus-like particles (VLPs). Located within a single CP subunit is an immunodominant epitope consisting of residues 169 to 180 [CP(169-180)], which is exposed on the surface of the subunit, but, in the structural context of the VLP, the epitope is buried and inaccessible to antibody. High levels of anti-CP(169-180) activity are associated with porcine circovirus-associated disease (PCVAD). The purpose of this study was to investigate the role of the immune response to monomer CP in the development of PCVAD. The approach was to immunize pigs with CP monomer, followed by challenge with PCV2 and porcine reproductive and respiratory syndrome virus (PRRSV). To maintain the CP immunogen as a stable monomer, CP(43-233) was fused to ubiquitin (Ub-CP). Size exclusion chromatography showed that Ub-CP was present as a single 33-kDa protein. Pigs immunized with Ub-CP developed a strong antibody response to PCV2, including antibodies against CP(169-180). However, only low levels of virus neutralizing activity were detected, and viremia levels were similar to those of nonimmunized pigs. As a positive control, immunization with baculovirus-expressed CP (Bac-CP) resulted in high levels of virus neutralizing activity, small amounts of anti-CP(169-180) activity, and the absence of viremia in pigs following virus challenge. The data support the role of CP(169-180) as an immunological decoy and illustrate the importance of the structural form of the CP immunogen in determining the outcome following infection.
Figures
Similar articles
-
Efficient expression and purification of porcine circovirus type 2 virus-like particles in Escherichia coli.J Biotechnol. 2016 Feb 20;220:78-85. doi: 10.1016/j.jbiotec.2016.01.017. Epub 2016 Jan 18. J Biotechnol. 2016. PMID: 26795354
-
Antibody responses following vaccination versus infection in a porcine circovirus-type 2 (PCV2) disease model show distinct differences in virus neutralization and epitope recognition.Vaccine. 2012 Jun 8;30(27):4079-85. doi: 10.1016/j.vaccine.2012.04.022. Epub 2012 Apr 19. Vaccine. 2012. PMID: 22521847
-
Replacing the decoy epitope of PCV2 capsid protein with epitopes of GP3 and/or GP5 of PRRSV enhances the immunogenicity of bivalent vaccines in mice.J Virol Methods. 2020 Oct;284:113928. doi: 10.1016/j.jviromet.2020.113928. Epub 2020 Jul 7. J Virol Methods. 2020. PMID: 32650038
-
The Carboxyl Terminus of the Porcine Circovirus Type 2 Capsid Protein Is Critical to Virus-Like Particle Assembly, Cell Entry, and Propagation.J Virol. 2020 Apr 16;94(9):e00042-20. doi: 10.1128/JVI.00042-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32075927 Free PMC article.
-
Structural roles of PCV2 capsid protein N-terminus in PCV2 particle assembly and identification of PCV2 type-specific neutralizing epitope.PLoS Pathog. 2019 Mar 1;15(3):e1007562. doi: 10.1371/journal.ppat.1007562. eCollection 2019 Mar. PLoS Pathog. 2019. PMID: 30822338 Free PMC article.
Cited by
-
Exploring the surface epitope and nuclear localization analysis of porcine circovirus type 3 capsid protein.AMB Express. 2023 Dec 8;13(1):141. doi: 10.1186/s13568-023-01652-6. AMB Express. 2023. PMID: 38066347 Free PMC article.
-
Generation of porcine circovirus type 4 virus-like particles and their use to detect serum antibodies.Arch Virol. 2024 Mar 7;169(3):67. doi: 10.1007/s00705-024-05997-6. Arch Virol. 2024. PMID: 38451379
-
The level of decoy epitope in PCV2 vaccine affects the neutralizing activity of sera in the immunized animals.Biochem Biophys Res Commun. 2018 Feb 12;496(3):846-851. doi: 10.1016/j.bbrc.2018.01.141. Epub 2018 Jan 31. Biochem Biophys Res Commun. 2018. PMID: 29374509 Free PMC article.
-
Porcine Circovirus 2 Manipulates the PERK-ERO1α Axis of the Endoplasmic Reticulum To Favor Its Replication by Derepressing Viral DNA from HMGB1 Sequestration within Nuclei.J Virol. 2021 Sep 9;95(19):e0100921. doi: 10.1128/JVI.01009-21. Epub 2021 Jul 21. J Virol. 2021. PMID: 34287039 Free PMC article.
-
Molecular Epidemic Characteristics and Genetic Evolution of Porcine Circovirus Type 2 (PCV2) in Swine Herds of Shanghai, China.Viruses. 2022 Jan 29;14(2):289. doi: 10.3390/v14020289. Viruses. 2022. PMID: 35215883 Free PMC article.
References
-
- Allan GM, et al. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 145:2421–2429 - PubMed
-
- Chae C. 2005. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 169:326–336 - PubMed
-
- Chae C. 2004. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet. J. 168:41–49 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

