Critical role for interferon regulatory factor 3 (IRF-3) and IRF-7 in type I interferon-mediated control of murine norovirus replication
- PMID: 23035219
- PMCID: PMC3503103
- DOI: 10.1128/JVI.01824-12
Critical role for interferon regulatory factor 3 (IRF-3) and IRF-7 in type I interferon-mediated control of murine norovirus replication
Abstract
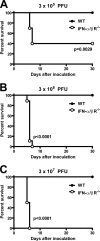
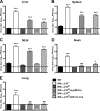
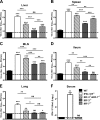
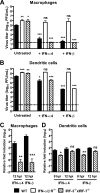
Human noroviruses (HuNoV) are the major cause of epidemic, nonbacterial gastroenteritis in the world. The short course of HuNoV-induced symptoms has implicated innate immunity in control of norovirus (NoV) infection. Studies using murine norovirus (MNV) confirm the importance of innate immune responses during NoV infection. Type I alpha and beta interferons (IFN-α/β) limit HuNoV replicon function, restrict MNV replication in cultured cells, and control MNV replication in vivo. Therefore, the cell types and transcription factors involved in antiviral immune responses and IFN-α/β-mediated control of NoV infection are important to define. We used mice with floxed alleles of the IFNAR1 chain of the IFN-α/β receptor to identify cells expressing lysozyme M or CD11c as cells that respond to IFN-α/β to restrict MNV replication in vivo. Furthermore, we show that the transcription factors IRF-3 and IRF-7 work in concert to initiate unique and overlapping antiviral responses to restrict MNV replication in vivo. IRF-3 and IRF-7 restrict MNV replication in both cultured macrophages and dendritic cells, are required for induction of IFN-α/β in macrophages but not dendritic cells, and are dispensable for the antiviral effects of IFN-α/β that block MNV replication. These studies suggest that expression of the IFN-α/β receptor on macrophages/neutrophils and dendritic cells, as well as of IRF-3 and IRF-7, is critical for innate immune responses to NoV infection.
Figures
References
-
- Au WC, Moore PA, Lafleur DW, Tombal B, Pitha PM. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210–29217 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

