Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes
- PMID: 23035643
- PMCID: PMC4337976
- DOI: 10.1111/tra.12016
Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes
Abstract
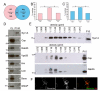
Wingless acts as a morphogen in Drosophila wing discs, where it specifies cell fates and controls growth several cell diameters away from its site of expression. Thus, despite being acylated and membrane associated, Wingless spreads in the extracellular space. Recent studies have focussed on identifying the route that Wingless follows in the secretory pathway and determining how it is packaged for release. We have found that, in medium conditioned by Wingless-expressing Drosophila S2 cells, Wingless is present on exosome-like vesicles and that this fraction activates signal transduction. Proteomic analysis shows that Wingless-containing exosome-like structures contain many Drosophila proteins that are homologous to mammalian exosome proteins. In addition, Evi, a multipass transmembrane protein, is also present on exosome-like vesicles. Using these exosome markers and a cell-based RNAi assay, we found that the small GTPase Rab11 contributes significantly to exosome production. This finding allows us to conclude from in vivo Rab11 knockdown experiments, that exosomes are unlikely to contribute to Wingless secretion and gradient formation in wing discs. Consistent with this conclusion, extracellularly tagged Evi expressed from a Bacterial Artificial Chromosome is not released from imaginal disc Wingless-expressing cells.
© 2012 John Wiley & Sons A/S.
Figures

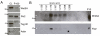
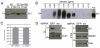
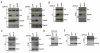

References
-
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. - PubMed
-
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. - PubMed
-
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11(6):791–801. - PubMed
-
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Current biology: CB. 2000;10(6):293–300. - PubMed
-
- Port F, Basler K. Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic. 2010;11(10):1265–1271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

