Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: study protocol for a randomized controlled trial
- PMID: 23035669
- PMCID: PMC3519528
- DOI: 10.1186/1745-6215-13-186
Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: study protocol for a randomized controlled trial
Abstract
Background: Many women try to stop smoking in pregnancy but fail. One difficulty is that there is insufficient evidence that medications for smoking cessation are effective and safe in pregnancy and thus many women prefer to avoid these. Physical activity (PA) interventions may assist cessation; however, trials examining these interventions have been too small to detect or exclude plausible beneficial effects. The London Exercise And Pregnant smokers (LEAP) trial is investigating whether a PA intervention is effective and cost-effective when used for smoking cessation by pregnant women, and will be the largest study of its kind to date.
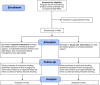
Methods/design: The LEAP study is a pragmatic, multi-center, two-arm, randomized, controlled trial that will target pregnant women who smoke at least one cigarette a day (and at least five cigarettes a day before pregnancy), and are between 10 and 24 weeks pregnant. Eligible patients are individually randomized to either usual care (that is, behavioral support for smoking cessation) or usual care plus a intervention (entailing supervised exercise on a treadmill plus PA consultations). The primary outcome of the trial is self-reported and biochemically validated continuous abstinence from smoking between a specified quit date and the end of pregnancy. The secondary outcomes, measured at 1 and 4 weeks after the quit date, and at the end of pregnancy and 6 months after childbirth, are PA levels, depression, self-confidence, and cigarette withdrawal symptoms. Smoking status will also be self-reported at 6 months after childbirth. In addition, perinatal measures will be collected, including antenatal complications, duration of labor, mode of delivery, and birth and placental weight. Outcomes will be analyzed on an intention-to-treat basis, and logistic regression models used to compare treatment effects on the primary outcome.
Discussion: This trial will assess whether a PA intervention is effective when used for smoking cessation during pregnancy.
Trial registration: ISRCTN48600346.
Figures
References
-
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Dev. 2003;75:21–33. - PubMed
-
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):125–140. - PubMed
-
- Royal College of Physicians. Smoking and the young. A report of a working party of the Royal College of Physicians. London: RCP; 1992.